
Malek Allababidi
Student Participant
IMED
Cardiology
Email:
Week 1 Heading link
In our first week of Clinical Immersion, we came across the variety of interesting procedures and cases that cardiology has to offer, and we highlighted many learning needs and potential future directions for our team.
We started in the Cardiology clinic. We got to know the general workflow, the layout of the clinic and labs, and we were introduced to the team. We followed our clinical mentor, Dr. Wissner, and interacted with several patients. Our next visit was to the Echo lab, where we observed a Transesophageal Echocardiography (TEE) procedure, and some stress tests too. The highlight of my week, however, was in the Electrophysiology (EP) and Cath labs, where we observed an ablation, a thrombectomy, and some angiographies. I was most fascinated by how they were able to generate complex and detailed 3D maps of patient hearts during ablations, and I see myself exploring this technology further.
Throughout the week, we also kept our eyes open for any good or bad designs in the hospital setting. I have identified some examples below:
Good design: Before any heart tissue is burned during an ablation, a 3D visualization of the patient’s heart is generated, and several variables such as voltages and conduction velocities are mapped across this 3D image. This way, lots of information is known about the patient’s heart, which allows the precise locations of abnormal signals to be identified for subsequent burning. This mapping feature is a good design as it increases precision since the cardiologist might otherwise burn excess heart tissue, which would increase the risk of complications.
Bad Design: During our clinical visit, we were shown models of Implantable Cardioverter Defibrillator (ICD) devices, which are typically used to shock a patient’s heart if it suddenly goes into ventricular tachycardia. There are two types: a traditional transvenous ICD, and a subcutaneous ICD. The subcutaneous ICD seemed to be too big, especially if it is to remain underneath a patient’s skin for the rest of their life. This is a bad design, as some patients may require a subcutaneous ICD due to special circumstances, and its large size can create discomfort for patients.
Week 2: Where's your sense of rhythm? Heading link

This week, we freely roamed the cardiology department, bouncing back and forth between labs and patients. Now that we are more oriented and comfortable in the procedural lab environment, we were able to interview many clinicians and learn a lot more about the procedures we were observing.
One particular procedure that I was intrigued by was the insertion of a loop recorder for a patient. I found it to be quite elegant, as it was neat, quick and easy. Implantable Loop Recorders (ILR) are devices that are implanted subcutaneously on the chest in order to monitor and record arrhythmias, and transmit this data to the cardiologist for review. ILR’s are typically indicated in conditions such as unexplained syncope or undocumented palpitations. I have provided a storyboard representation of the ILR insertion procedure and its “pain points” below.
Storyboard:
1. The patient is prepped under sterile conditions.
Pain points:
- Patient is awake, not sedated, and may move around.
2. Lidocaine is injected through a large needle into the site of ILR insertion.
Pain points:
- Patient might feel pain/pinch.
3. A cutting tool is used to make an incision underneath the skin.
Pain points:
- Patient feels pressure on their chest.
- Patient can bleed a lot.
4. The ILR comes pre-loaded in a loading tool. The loading tool is inserted into the incision, flipped, and then a plunger is used to push the ILR in through the incision and underneath the skin.
Pain points:
- Patient has to stay away from magnets, as they can disrupt the ILR.
- Patient will be sore for a couple of days.
5. The loading tool is removed, and the physician applies pressure to the wound site until the bleeding stops, then applies a sterile patch and dresses the wound.
Pain points:
- Patient may bleed a lot.
6. Device representative/technician programs and calibrates the ILR with parameters specific to the patient.
Pain points:
- Done through Bluetooth after insertion, so connection between programmer and device can be faulty or lost.
- Can take a while.
7. Patient receives a monitor to plug in at home, which collects data from the ILR and transmits information to the cardiologist for review every night.
Pain points:
- Monitor cannot be near any phones.
- Monitor always needs to be plugged in and on.
In the ILR insertion procedure we observed, the patient had been experiencing episodes of unexplained syncope, and so the cardiologist wanted more information about the patient’s heart rhythm in order to identify possible causes for the syncope. Knowing this, I searched the literature for more information about ILR’s and their indications, and I have summarized a review article I came across below.
Kwok et al. have provided a review of the different uses and indications of ILR’s, as well as their issues and how they are used in different settings. They describe that the established indications for ILR’s are for the management of transient loss of consciousness, and the diagnosis of undocumented palpitations. ILR’s can also be used to monitor atrial fibrillation, and for risk stratification in patients with myocardial infarctions and inherited cardiomyopathies, though these indications are less established.
ILR’s will record cardiac electrical activity during symptomatic episodes and when asymptomatic arrhythmias are detected. They are advantageous in that this electrical activity can be recorded while patients are going about their daily lives, rather than in a hospital setting. They also record over much longer periods of time, unlike cardiac tape recorders which record for shorter times at which symptoms or an arrhythmia might not occur. However, ILR’s still have some limitations. For example, the device itself and its equipment might be costly for the patient. In addition, there is also a risk of pain, bleeding, infection, and detection of false-positive arrhythmias, though these risks are small.
The authors conclude that ILR’s are very valuable diagnostic tools for patients with both cardiac and non-cardiac conditions. However, they state that the risks and costs should be compared to the benefit of ILR’s for every patient, especially when cheaper alternatives are available, as patient conditions can vary.
Kwok CS, Darlington D, Mayer J, et al. A Review of the Wide Range of Indications and Uses of Implantable Loop Recorders: A Review of the Literature. Hearts. 2022;3(2):45-53. doi:10.3390/hearts3020007
Week 3: Everybody needs a need Heading link
Our third week of cardiology was yet again filled with exciting new procedures, techniques and events. We continued to probe the cardiologists with more questions about why they do what they do throughout their procedures, and we even had a chance to brainstorm some problem spaces together.
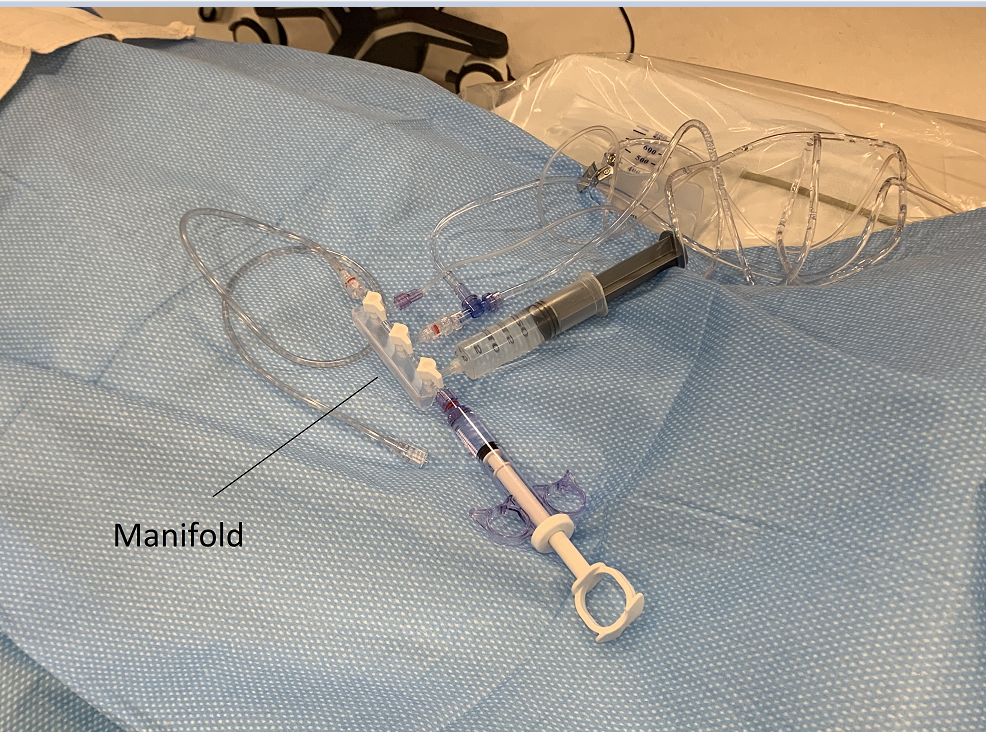
Our focus for this week was to begin formulating needs statements based on our observations. In doing so, we had a conversation with a general cardiology fellow who provided us with an interesting problem. He described to us how crucial it is to avoid introducing bubbles through the cath lab manifold and syringe set-up, as even a small amount of air can embolize and lead to a myocardial infarction (MI) in the patient.
In order to learn more about this problem space, I searched the literature for more information. According to a case report and literature review by Dib et al., air emboli are complications of cardiac cath procedures that result from iatrogenic introduction of air bubbles into the bloodstream. Though air embolization is an uncommon complication with an incidence of 0.1-0.3%, they can occlude blood vessels and cause ischemia, which can lead to cellular damage, significant morbidity, and even death.
Air bubbles are prevented with careful aspiration of catheters, and thorough flushing of coronary equipment. Our team had also observed the cardiologists vigorously tapping the manifold set-up, which we were later told was “second-nature”, but still tedious. Thus, it is clear that there is a need for a more meticulous method to prevent this complication. I have developed some iterations of a needs statement below, keeping in mind the population, opportunity and outcome (“POO”).
Needs Statement 1:
A way to prevent air bubble formation during cardiac catheterization procedures.
In this first iteration, I simply phrased the problem, without the population or outcome. The next iteration will need to include who will be involved in the picture, and the impact of addressing the need.
Need Statement 2:
A way for cardiologists to prevent air bubble formation during cardiac catheterization procedures, in order to reduce complications.
This second iteration outlines the problem, opportunity and outcome, but is too broad and general. For example, as I mentioned earlier, air bubbles can be prevented by using work-around practices like flushing equipment and vigorously tapping throughout the procedure, but this process is tedious. Therefore, this statement does not fully capture the need. In addition, the outcome is also too broad, as many different complications can arise throughout any kind of procedure.
Need Statement 3:
A way for cardiologists to prevent air bubble formation during cardiac catheterization procedures without having to use work-around practices, in order to reduce the incidence of iatrogenic catheterization-related MI.
In the third and final iteration, I have specifically identified the outcome to be measured upon addressing the need. I have also expanded upon the opportunity, in order to narrow the scope further.
Dib J, Boyle AJ, Chan M, Resar JR. Coronary air embolism: A case report and review of the literature. Catheterization and Cardiovascular Interventions. 2006;68(6):879-900. doi:10.1002/ccd.20880
Week 4: Solve this, Patent that Heading link
Our focus this week was to continue identifying more needs and brainstorming problem spaces, while also starting to think of existing patents and commercial solutions. Throughout this process, however, I could not help but to think about a problem we had been introduced to earlier: cardiac pacemaker replacements.
According to the electrophysiologists (EP’s) we observed, cardiac pacemakers typically have a battery-life of about 6-10 years, and need to be replaced after this time. This process is invasive, as it involves the removal of the old pacemaker and the implantation of the new one, though the lead wires are kept in place in the heart. Because of this invasive nature, infection can occur in the patients. For example, Klug et al. reported a 2-fold increase in infection in patients with pacemaker replacements compared to patients with de-novo implants.
Knowing this, I wondered about the possibility of eliminating the need for a replacement surgery, and so my team and I proposed the following needs statement: Electrophysiologists replacing pacemaker batteries need to identify alternative ways to replace pacemaker batteries that reduce infection related complications.
I searched patent databases for attempts to address this need and subsequently came across a U.S. Pat. No. 2007/0270916A1, in which Fischell et al. describe a rechargeable pacemaker design that can also be applied to other implantable medical devices such as ICD’s. Most importantly, they claim a device that can be recharged externally through magnetic induction, through the use of a coil that is placed within a plastic header near the surface of the device. They claim that this recharging process can be done using an external power cord, or a portable recharging device that would also allow mobility during recharging. Moreover, they also claim an antimicrobial coating on the device and its wires that would slowly release over time, in order to further reduce infection complications.
This patent would address the need described above, as it allows the battery of a pacemaker to simply be recharged rather than having to be replaced entirely through an additional surgical procedure. However, I recall a conversation I had with an attending EP for a pacemaker implant procedure that we observed, in which he described to me that many attempts at redesigning the cardiac pacemaker did not make it to market. We discussed the ideas of wireless and rechargeable pacemakers and their limitations, and how a medical device company would not be able to sell you another pacemaker if it was rechargeable. But we also talked about a much more significant limitation, which is the fact that pacemakers are rapidly advancing and evolving, coming out with new features and specifications that a recharging capability might not be able to keep up with. For example, if a patient were to rely on a rechargeable pacemaker for their lifetime, then they would never receive the newest technology, and this has been the biggest drawback for bringing the rechargeable pacemaker to fruition.
Thus, it seems that this need is a lot more complex than we had anticipated, with many different factors and stakeholders to consider. Yet, I am still convinced of its validity, and I wonder, will pacemaker technology ever reach a limitation that necessitates a more substantial longevity?
Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116(12):1349-1355. doi:10.1161/CIRCULATIONAHA.106.678664
Fischell RE, Johnson KM. Cardiac pacemaker with integrated battery. Published online November 22, 2007. Accessed July 24, 2022. https://patents.google.com/patent/US20070270916A1/en?q=rechargeable+pacemaker&oq=rechargeable+pacemaker
Week 5: Money talks Heading link
In week 3, I described a unique problem that our team had observed in the cardiac cath lab, and I proposed a needs statement for this problem space. I have slightly revised this needs statement based on feedback and provided it below:
A way for cardiologists to eliminate air bubbles during cardiac catheterization procedures, in order to reduce the incidence of iatrogenic catheterization-related MI.
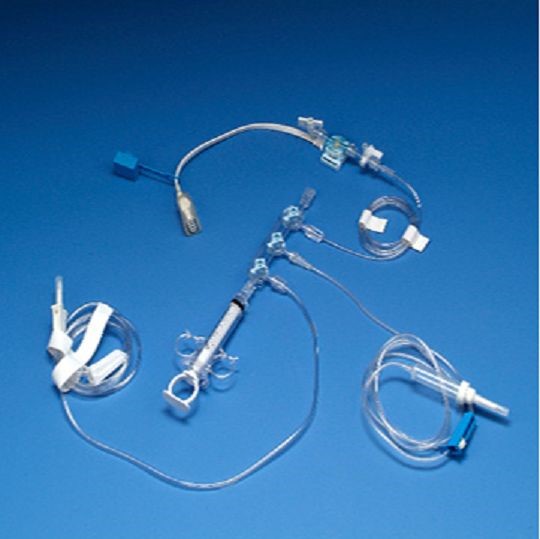
As I described in my week 3 blog post, preventing the introduction of air bubbles into patients during cardiac catheterization (cath) procedures is crucial, as these air bubbles can embolize and occlude coronary blood vessels, which could lead to an MI in the patient. After interviewing the interventional cardiologists in the cath lab, we learned that these air bubbles are currently eliminated simply by tapping the tubing and manifold setups, and by thoroughly flushing equipment. They described to us how these practices have become “second-nature” for them, and how they do not use any particular devices to eliminate air bubbles.
Our task for this week was to calculate a Total Addressable Market (TAM) for our needs statement, which is the overall revenue opportunity that is available to a product or service if 100% market share was achieved. I was curious about the TAM that an alternative solution to the aforementioned needs statement could potentially target, and I have outlined this calculation below. Since the air bubbles occur within tubing and manifolds used throughout the procedure, I will designate a standard manifold kit as the product.
TAM is calculated as the number of units per year for a product or service multiplied by the cost of this product/service.
According to a report from the American Heart Association (AHA) by Mozaffarian et al., approximately 1 million diagnostic cardiac catheterization procedures are performed annually. This would be the number of units per year.
A standard manifold kit costs approximately $200. This would be the cost per use, per cardiac cath procedure.
Using the above numbers, the TAM can be calculated as:
TAM = 1,000,000 * $200 = ~ $200 million
Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics – 2016 Update. Circulation. 2016;133(4):e38-e360. doi:10.1161/CIR.0000000000000350
Standard Manifold Kits. Rehabmart.com. Accessed July 31, 2022. https://www.rehabmart.com/product/standard-manifold-kits-39752.html
Week 6: Done in a heartbeat Heading link
My immersion in cardiology this summer was highly rewarding. I had the privilege of witnessing the most sophisticated and advanced technologies, as well as the most unique cases in cardiology. Between clinic, the cath lab, and the EP lab, my experiences were always new and exciting. When I first began my immersion, I wanted to apply and connect my medical knowledge to the clinical cases I was witnessing, and I was eager to get a more immersive view into what my future medical career might look like. However, soon enough the many pain points of the medical procedural environment revealed themselves, and I therefore returned to my engineering roots. Alongside my team, I probed the medical personnel with questions and ideas, and suddenly my notebook was filled with problem spaces and areas of improvement.
My first lesson learned is that nothing is perfect, no matter how complex and sophisticated it seems. At first, it took time to understand the nuances of catheters and ablation, and why everything was done the way it was. But in doing so, we were able to identify issues in almost every procedure we observed. Of course, without doing these procedures ourselves, we could never completely understand all of the intricacies of every step, yet every observation still presented a unique problem to think about. This was why the different staff we interviewed had different outlooks on what needed to be improved and innovated, and sometimes this did not align with our own team’s goals. The trick was not to branch off of one individual’s experiences, but to put them all together to get an idea of the whole picture.
For future CIP members, I recommend standing close to the physicians and the rest of the medical personnel, and truly soaking in all of the clinical experience you get. Ask what, ask how, and ask all of the why’s. Most importantly, ask to wear the lead apron to get close to the patient bed!