Dionna Bidny
Student Participant
MS2
Neurosurgery
Pronouns: She/Her/Hers
Week 1: Sometimes light switches are more confusing than surgical tech Heading link
Medical education is funny. One moment I’m spending a solid 90% of my life on my couch with my nose in First Aid, and the next I’m a slightly sheepish fly on the wall in a neurosurgery OR surrounded by over 20 people performing 20 different roles. On one hand, I’m overwhelmed by the rapid onset of fast-paced and real-world medicine, the acute tangibility of it feeling incredibly palpable after a year of almost exclusive book learning. On the other hand, I feel a sense of calm and adventure. I get to be in an exciting space that reminds me why I’m here—long before the clinical responsibilities of third year set in. Additionally, I’m not here as a med student only, but also as an innovator. Of all the 20 different people and their 20 different roles in the OR, ours is not just to observe and absorb, but to analyze. What is working well? What is not? Here is one example each of a good design and a bad design I saw this week, as I adjust to a new pace and a new environment.
GOOD DESIGN: One particular tool stole the show in terms of an example of straightforward yet efficient design. The StealthStation by Medtronic is a brilliant device that allows real-time location of tumors, masses, foreign bodies, and truly anything else that is CT imageable. StealthStation is an image guided surgical system, that uses preoperative images to map to operative tools in real time. The system uses motion capture of reflective markers to first home the body area of interest to preoperative 3D scans, and based on known relationship between the scans and imaging, the system can then map reflective operative tools to CT image slices. Thus, the StealthStation display is able to accurately show the slice of interest based on operative tool location, as well as display that tool itself. This simple but ingenious process allows for real time locating of various imageable points of interest, ensuring tool accuracy. In the OR this week, we saw this system utilized for a tumor resection as well as a bullet removal. The integration of radiology, motion capture, and surgical real time application into a useful and user-friendly system demonstrated the value of interdisciplinary innovation in the OR.
BAD DESIGN: Truly, much of what we saw this week were beautifully choreographed systems and high efficiency tools. That being said, there are always gaps, and our perception of them will continue to grow as we develop a better understanding of our environment. One small but mighty gap was the location of the main light switches in the OR: outside the room, for no apparent reason. This led to a few system troubles. In neurosurgery, intraoperative imaging is highly common, and occasional room dimness helps enhance viewing of the numerous displays, particularly scopes and ultrasounds. Not only would frequent calls of “someone turn off the lights” sometimes go unheard and thus need repeating, but also a decent amount of time was necessary for someone to exit the room, change the light, and return. Is this a risk to patient or practitioner safety? Not directly. Could this process go far more smoothly to possibly help maintain provider focus? Absolutely. While we suspect there may have been a design criterion for this switch location, we were unable to elucidate that reason from interviews. Perhaps this suggests a larger scale issue with monitor visibility in the operating room.
Week 2: In which we were left to our own devices in Neuro ICU Heading link
“I have meetings this week; go hang out in the Neuro ICU” were essentially our prime instructions during week 2 of neurosurgery immersion. And thus we found ourselves on 6 am rounds with a group of neurosurgery residents, followed by more rounds with neurology later that morning. The experiences were both sobering (hearing a patient being informed of a terminal diagnosis) and enlightening (getting quizzed about medication mechanisms by the neurology attending, having to admit to him that No, we have not yet studied neuro block, but Yes, I somehow remember how Broca’s and Wernicke’s aphasias present from psychology in 8th grade).
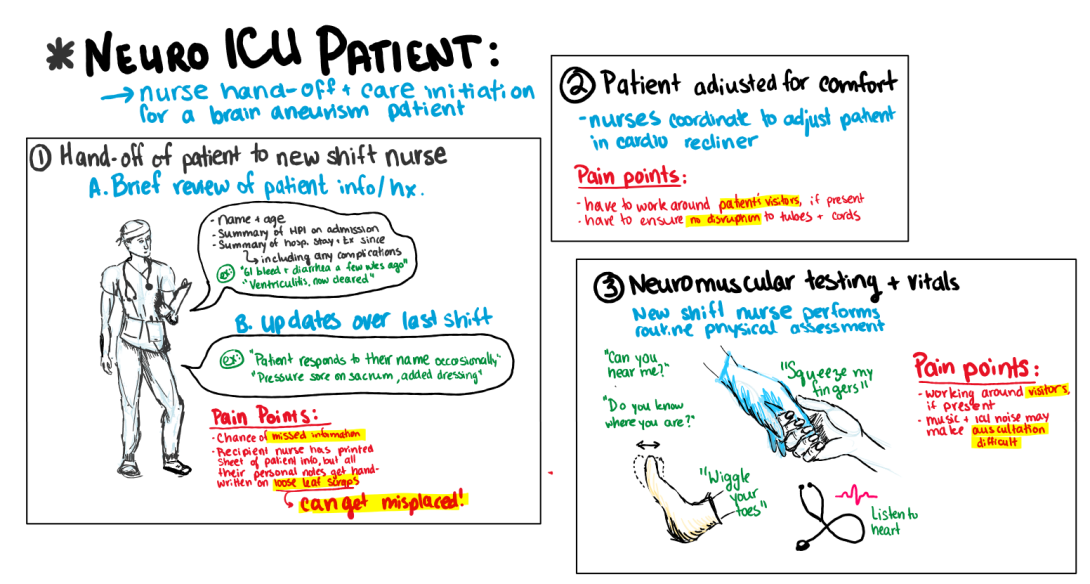
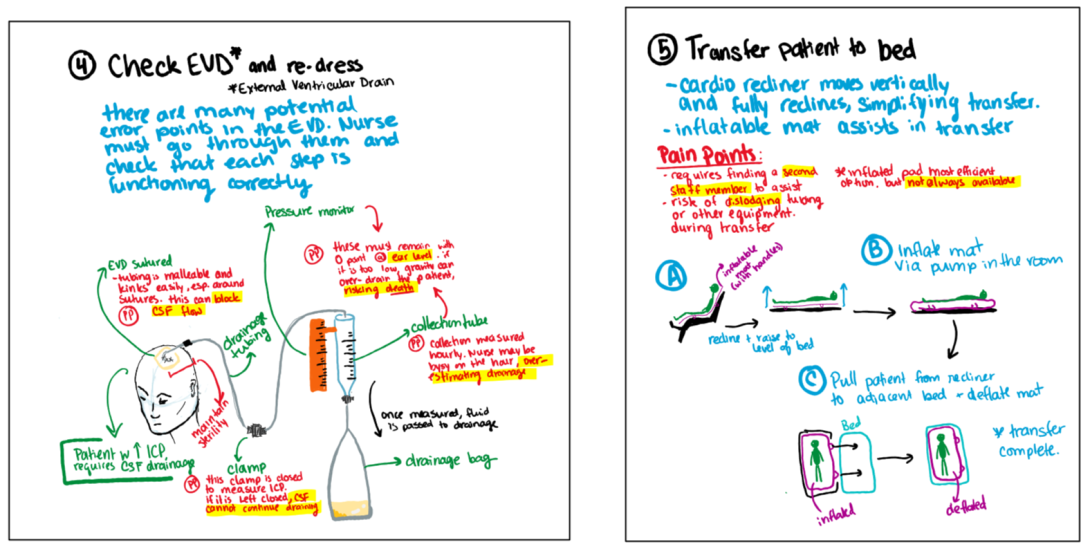
Later that week, another teammate and I had the great opportunity of shadowing a Neuro ICU nurse as she took care of one of her patients. So far, this was the most hands-on experience we had in the ICU, and the amount of details nurses have to keep track of even on an “easy” patient elucidated to me how many steps could go wrong even with the most attentive practitioner. One particular device, the external ventricular drain (EVD) stood out as a simple but highly protocol dependent device. EVDs are cerebrospinal fluid (CSF) drains for patients with increased intracranial pressure (ICP). The device drains excess fluid, and also measures ICP when the drain is momentarily closed. Errors can be fatal– leading to draining blockage, or over-draining. This is particularly a risk when the patient has a thicker CSF or a EVD blockage, in which drainage parameters must be constantly adjusted (and thus, monitored). With our nurse mentor casually chatting to us about EVD horror stories from “before her time” that are now used for training purposes to remind newer nurses of what not to do inspired me to storyboard the process we witnessed as the nurse took care of her patient. While the EVD and its process is the star of the show, I wanted to storyboard around it as well, highlighting the multiple steps in the few hours of patient care we saw, all the way from the nurse shift change to transferring the patient comfortably back to bed.
Shortly after, I found an insightful review paper that highlighted to me not only the range of possible EVD complications, but their significant prevalence. While these complications extend beyond the possible human error discussed here, increased overall complications lead to increased monitoring burden on the nurses and thus a greater chance of human error and further compilation. I explore this review paper further at the end of this post.
literature Heading link
In their 2020 review paper, Pierre-Louis et. al. explore the prevalence and type of EVD complications in neurosurgery patients. EVDs are one of the most common neurosurgical procedures, but the rate of related compilations is a cause for concern (EVD related hemorrhage occurs in about 41% of cases). The paper reviewed 12 total articles, narrowed down from literature searches based on inclusion of a) neurosurgical patients b) EVD use and c) documented complications. The authors found that the most common complications included aneurysm rebleeding, hemorrhage, meningitis, and EVD-related infections. Additionally the paper emphasized the consistent documentation of fluctuations of serum sodium levels and EVD duration as risk factors. The paper emphasizes a significant limitation: minimal research in the area of EVD complication, and a lack of consistency in the findings. The authors also claim that such complications are largely underreported. This is consistent with my own search. Based on what our nurse-mentor told us of errors and complications that have occurred even in our own hospital system, there appears little of such complications and errors reflected in the literature. It is thus difficult to discern if this is due to such complications being not widespread, or rather understudied. Piere-Louis et. al. suggest that perhaps it is in fact the latter.
Pierre-Louis, M., Alam, A., Stalin, K. S., Bhagia, M., Adereti, C., & Chacon, D. (2022). External ventricular drain complications in neurosurgery patients: A systematic.
Week 3: A broader perspective Heading link
Innovating within neurosurgery is hard.
Surrounded by advanced tech, as well as the company reps whose sole job it is to improve that tech, I am made to wonder if I even have a role here. Additionally, even when we do spot something that could be improved, highly trained and skilled physicians and nurses often tell us it’s not a problem; they manage just fine. On top of it all, neurosurgery and neuro ICU patients are often so complex that, even in larger scale literature, it is hard to pinpoint where complications even arise from. How do we innovate on anecdotal evidence from the perspective of one event, one inexperienced student, one story?
I have been incredibly conscious to not make the dangerously easy mistake of inventing problem spaces where they don’t exist, simply for the sake of task completion.
That being said, my role is to look towards a broader perspective. It is to examine not just the interaction between individuals and their tech, but also to ask questions about variables regarding the hospital as a whole: costs, lengths of stay, practitioner burden and burnout.
Last week I discussed External Ventricular Drains (EVDs), and when considering this space, I have gone through all these mental gymnastics. The literature is meager due to the difficulty of isolating variables. Nurses are skilled and state they do not consistently run into issues they can’t fix. Patients are complex, regardless. So I stepped back: How much do EVD replacements cost? What are the main complications that lead to EVD replacements? How much do these complications increase length of stay? With this perspective shift, I was onto something.
The first iteration of my needs statement in this space was as follows:
- A method to decrease complications in EVD patients in order to decrease cost and monitoring burden
A 2020 review paper by Aten et. al. elucidated to me that the primary and most targetable cause of EVD complications was drain obstruction, thus developing a more targeted scope from a dreadfully broad problem. This led to my second iteration:
- A method to decrease drain obstruction in EVD patients in order to decrease cost and monitoring burden
Finally, this paper also emphasized that the average EVD patient has 1.26 EVDs placed, highlighting a high replacement rate and high cost burden for the hospital, and subsequent increase in length of stay for the patient. These metrics are easiest to measure and utilize as representations for EVD complication (rather than something non specific as monitoring burden). With this in mind, I iterated my needs statement one more time:
- A method to decrease drain obstruction in EVD patients in order to decrease cost of care and duration of stay
I don’t know if we will stick to this problem space, ultimately. But it has been an impactful exercise in recognizing my role outside of a direct clinical interaction. I’m starting to see the bigger picture.
Aten, Q., Killeffer, J., Seaver, C., & Reier, L. (2020). Causes, complications, and costs associated with external ventricular drainage catheter obstruction. World neurosurgery, 134, 501-506.
Week 4: More information, more confusion Heading link
The more I know the more I realize I don’t know.
A theme of life, frankly.
Four weeks through Clinical Immersion, and we are still unsure what space to focus on. There has been so much learning and growt,h but with that comes confusion and new avenues to consider.
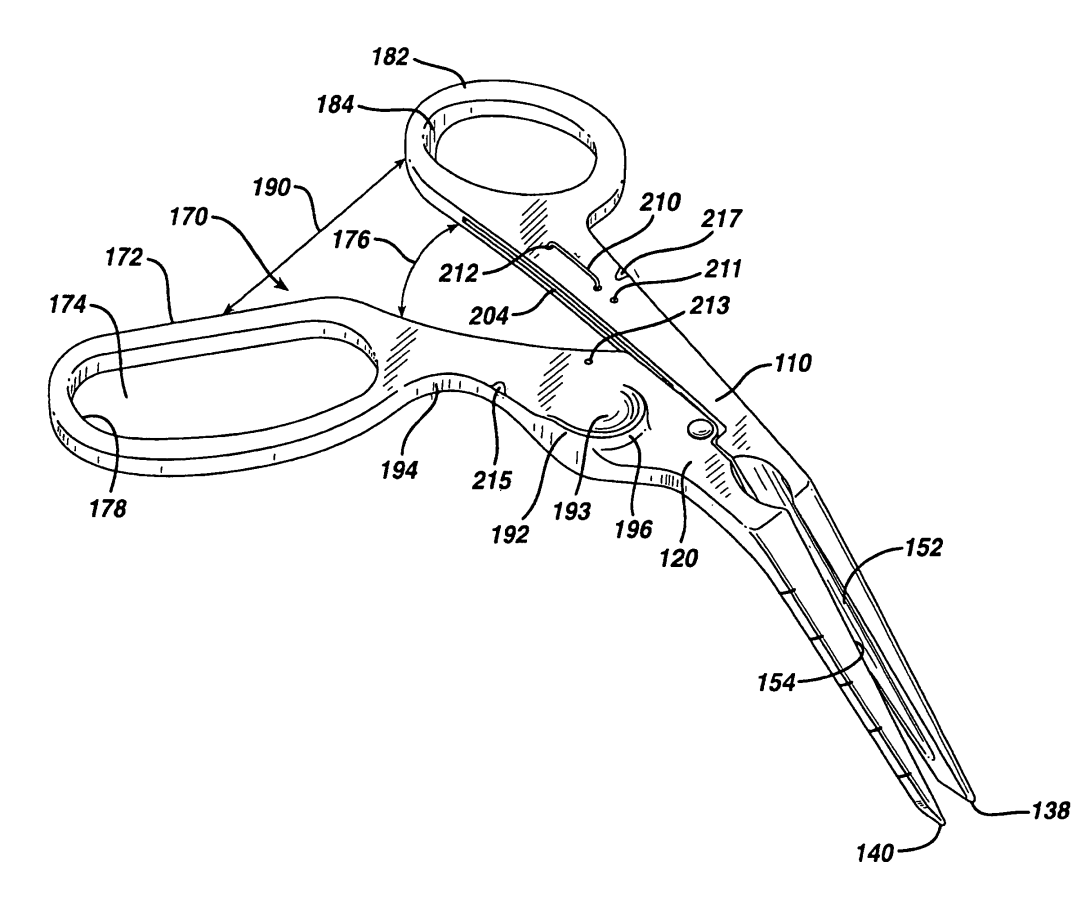
Over the last two weeks especially, we got to observe surgeons of different body types and statures sharing a case. The table height was always adjusted to the leading surgeon, and when the hight difference was drastic, the secondary surgeon would look uncomfortable or have awkward leverage to hold devices. This got us thinking about surgeon ergonomics– so often tools and devices are focused on patient safety and outcomes, with minimal attention paid to surgeon comfort and longevity. Numerous articles express that surgeon ergonomics is an understudied field, and that solutions exist within an interdisciplinary space of educational training and environmental optimization (Gadjradj et. al., 2020; Marvounis et. al., 2021; Soueid et. al., 2010). While a multifactorial problem (and solution), we are considering tackling this problem from the branch of device ergonomics. Many of the previously mentioned articles discussed that often only the “working” tip of OR instruments are optimized, and the griping end is often one size fits all. Laparoscopic instruments have gotten significantly more attention than open surgery instruments as they are constantly being gripped. In the OR watching open surgery, I saw how many scissors or retractors looked awkward to hold. That being said, narrowing down on a space of innovation still proves difficult, due to the variety of and exchange of tools in the OR. Patents were equally difficult to find due to this non specificity.
One 2011 patent by Grayzel et. al. (“Ergonomic Hand Instruments) explores the design of a hand instrument designed specifically for percutaneous entry of a blood vessel.
The first claim describes the angle of the “scissor” handles with respect to the cutting tip, and explains that this allows the user to press against a forefinger control surface near the thumb hole to have better pivoting stability accuracy of vessel entry during surgery. The thumb hole is also larger than on standard surgical scissors allowing for more adjustable positioning. Subsequent claims describe designs with slightly different angles to the control surface and handles, as well as different positions for the forefinger control surface.
While this does not directly apply to what we see in the OR, the design reflects the close relationship between easy surgical access and improved grip position. Designing with both hand position and surgical goal in mind minimizes the need for awkward positioning and subsequent discomfort.
Gadjradj, P. S., Ogenio, K., Voigt, I., & Harhangi, B. S. (2020). Ergonomics and related physical symptoms among neurosurgeons. World neurosurgery, 134, e432-e441.
Mavrovounis, G., Meling, T. R., Lafuente, J., Fountas, K. N., & Demetriades, A. K. (2021). Postural ergonomics and work-related musculoskeletal disorders in neurosurgery: lessons from an international survey. Acta neurochirurgica, 163(6), 1541-1552.
Soueid, A., Oudit, D., Thiagarajah, S., & Laitung, G. (2010). The pain of surgery: pain experienced by surgeons while operating. International Journal of Surgery, 8(2), 118-120.
Week 5: Strength in numbers Heading link
What do you do when you can’t decide between two potential innovation spaces?
Combine them into one, of course.
Over the last 5 weeks we have investigated surgical tech, ICU devices, surgeon experiences and comfort. We have talked to multiple stakeholders, and considered numerous problem spaces. As we started this week, two areas stood out to us as top contenders around which to innovate: The space of surgeon ergonomics as discussed last week, and the Mayfield skull clamp.
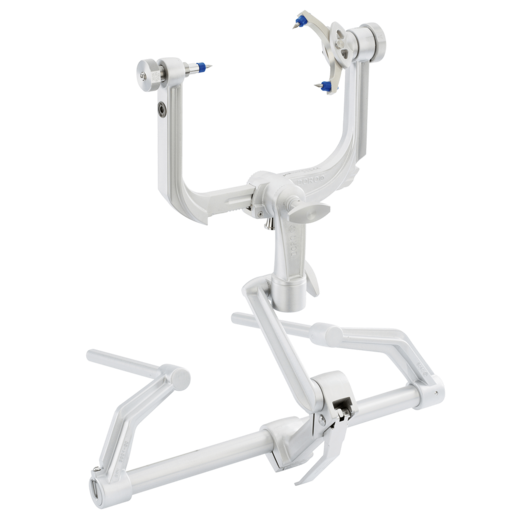
The Mayfield clamp, while not previously discussed on this blog, has been a point of discussion in my team since week 1. The clamp is a 3 point fixation device that is used to hold the skull and cervical spine stable during cranial and cervical surgeries, while also ensuring the face is not compressed during prone procedures (see image). The pins are pressed into the skull with 80lb of force, and the clamp is secured to the surgical bed by a modular arm.
The Mayfield clamp is inexpensive, durable, and provides excellent fixation. However, significant documentations exist regarding complications, including pin lacerations, vascular injury, and even (rarely) skull fracture in vulnerable patients. Additionally, armed with our new considerations of surgeon comfort and ergonomics, we noticed that the placement of the clamp was cumbersome: While the multiple degrees of freedom in the support arm (see image) allow for careful case-specific orientation, holding the patient head and rest of the construct stable as each portion is fixed into place required numerous hands, awkward positioning, and frequent double checking. Combining the space of Mayfield skull clamps with ergonomics, we developed a new working needs statement:
- Surgeons preparing for cranial and cervical spine procedures face challenges in positioning and stabilizing the head and require an ergonomic and secure way to reduce patient complications and increase ease of surgeon usability.
Exploring Mayfield clamps as a market has proven difficult. The clamps are used for many (but not all) cervical and cranial procedures, and are also highly reusable devices with a potentially >10year lifespan (challenging an accurate per-patient cost estimation).
For an initial market estimate, I searched the Agency for Healthcare Research and Quality database (found at https://datatools.ahrq.gov/hcupnet-dua) for frequency of relevant procedures in the US. The data seemed limited, only elucidating craniotomies and cervical procedures and ending in 2007. But given this limitation, the annual patient population was 240,000 annual cases across the US in 2007. multiplying this number with an estimate of $50 per patient cost results in an existing 12M total market value in the US. This number is likely an underestimate due to data access limitations and potential variations in case numbers between 2007 and now, but gives us a preliminary picture of the market space in which we are working. Innovating within this space to increase efficiency, decrease surgeon discomfort, and decrease complications would also cumulatively save costs related to OR time, patient care, and surgeon physical limitations or need for time off.
Week 6: Just the beginning Heading link
In 2019 as a bright-eyed biomedical engineering undergrad at UIC, I participated in a previous iteration of the Clinical Immersion Program. My experience with innovation through the program had been so positive that my last blog post was jokingly titled, “In which I give up my medical school dreams because I want to be an engineer forever.”
And yet, here I am, more confident than ever in my medical career choice–not because I changed my mind since 2019, but rather because I continue to discover how deeply innovation, engineering, medicine, and the other disciplines that have shaped me as a scholar can coexist. I’ve always “known” this, certainly, but the countless experiences (including CIP 2022) that I have had within academia continue to exemplify to me that I do not have to give up one part of me to let the others flourish. Our proposed project space of reimagining surgical head clamps has been met incredibly positively, and I thoroughly look forward to continuing collaborating with engineers as the project flows through senior design. I am grateful to CIP for continuing to guide me in my place in medicine, engineering, and beyond.
pic Heading link
CIP 2022
About
Student doctor, biomedical engineer, and musicologist with a drive for the interdisciplinary. I aim to blend my knowledge in user-centered technology development, music, and healthcare to forge discoveries in performing arts rehabilitation medicine with an emphasis on disability access and long term care.
Academic and professional interests: Performing arts and sports medicine, rehabilitation tech, disability access, interdisciplinary innovation