
Julie Gawenda
Student Participant
Pronouns: She/Her/Hers
About
Introduction: My name is Julie Gawenda and I am a 4th-year undergraduate student, majoring in biomedical engineering and minoring in biological sciences, on the pre-medicine track. I hope to gain an understanding of the day-to-day life of a physician through this immersion, and along with my group members, develop a meaningful contribution to the ophthalmology department. Whether it is through transformative or incremental innovation, I see the potential for a joint effort between BME and IMED students to bring great change to their respective medical departments, and I am curious to see what these 6-weeks will lead us to develop!
Area of Research: Department of Ophthalmology; Dry Eye & Ocular GVHD Clinic | Lions of Illinois Eye Research Institute
Mentor: Dr. Sandeep Jain, Director of the Dry Eye & Ocular GVHD Clinic at UI Health, Assistant Professor of Ophthalmology at the University of Illinois College of Medicine
Contact Information: jgawen2@uic.edu | www.linkedin.com/in/julie-gawenda/
Week 1: Donning Our Engineer Thinking Caps (Good vs. Bad Designs)
June 27th—July 1st, 2022
My Bread & Butter
This past week, the annual collaboration between Innovation Medicine (IMED) second-year medical school students and senior biomedical engineering (BME) students began, ending the two-year pause of the program following limitations posed by the COVID-19 pandemic. I distinctly remember first hearing about the Clinical Immersion Program (CIP) in my freshman year of college (Fall 2019), with Dr. Felder introducing the concept of a “clinical immersion” and identifying potential medical innovation needs in my BIOE 101: Introduction to Bioengineering class. My very motivation for majoring in biomedical engineering stemmed from an interest in innovating existing medical techniques to match the rapid turnover and development of new technologies. Thus, CIP sounded exactly like my bread and butter, an opportunity to enter a medical department and propose a change–as a powerful collaboration between M2s and senior BMEs.
Delving headfirst into the Department of Ophthalmology and shadowing Dr. Jain, the director of the Dry Eye & Ocular GVHD Clinic at Lions of Illinois Eye Research Institute, I wasn’t quite sure what to expect. Based on the title of the clinic alone, I was sure to encounter eyes—the proposition which both fascinated and dismayed me, as I was sure the experience would “make or break” the field of ophthalmology for me. Shadowing at the clinic depicted me scrawling down acronyms (OD – oculus dextrus or right eye, OS – oculus sinister or left eye), viewing manifestations of the same disorder in different patients, and seeing a spectrum of iris colors and inflammation of the corneal epithelium through the view scope of a slit microscope. It was not only bread and butter; there was jam (an OCULUS keratograph), honey ( a “pregnancy-test”-like InflammaDry), and Nutella (confocal microscopy imaging). Simply put, there was so much more than I expected, addressing ocular issues that dramatically influence one’s quality of life.
Our group was tasked with observation; looking for a potential medical need that can be satisfied by innovation on our behalf. Yet in the midst of looking for medical needs, I found myself rapidly picking up bits of ophthalmological knowledge, more complex than the Latin abbreviations for the eye. Dr. Jain, Christine, and the other lab technicians frequently engaged us in conversation, helping us process what we would pick up from patient conversations and research done after clinic hours. Oftentimes, our discussions would bring more questions than answers, as in the chicken and egg scenario of does a lack of sleep aggravate/cause dry eye syndrome or does dry eye syndrome cause a lack of sleep? We would learn how to identify patient’s past eye procedures based on corneal epithelium thickness mapping alone, as patients who have had LASIK would have thicker epithelium centrally, while patients with dry eye syndrome may have thinning superiorly. With a firmer grasp of ophthalmology and the functioning of the clinic, I was able to identify some of the following good and bad designs.
Good Design: Design and Flow of Rooms in Clinic
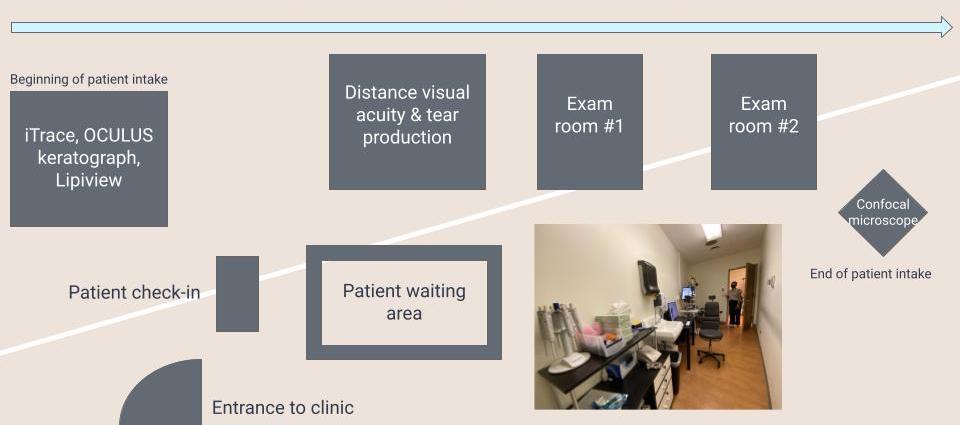
Traditionally, exam rooms in ophthalmology clinics are rather small, and this stands the case at the Dry Eye & Ocular GVHD Clinic. However, not only is the space within the rooms maximized, with all the equipment aligned along the right wall, creating a walkway on the left but the flow of the rooms themselves are streamlined from left to right. For new patient in-take, once the patient is checked in, they will be ushered into the leftmost room which contains 3 machines (iTrace, OCULUS keratograph, and Lipiview) serving as various tools that will help Dr. Jain come to his clinical diagnosis. Next, the patient will enter the adjacent room to the right to assess distance visual acuity and tear production, after which the patient will be admitted to one of the adjacent exam rooms, to be examined with the slit microscope by Dr. Jain. Afterward, confocal microscopy imaging takes place outside of the exam rooms, thus maintaining a flow of patient intake from left to right across the expanse of the clinic.
Bad Design: Blue-Dye Stained Eyes
One of the tests performed in the clinic on almost every single patient, regardless of whether it is a new patient intake or follow-up, is a lissamine eye stain. Lissamine is a blue dye that when applied to the surface of the eye and focused on by a slit microscope, can stain patches of dryness on the epithelium. However, most of the patients in Dr. Jain’s clinic will walk out of the clinic, despite a saline rinse by a technician, with blue-stained eyes, lasting between 3-4 hours. Whilst this is something that most of Dr. Jain’s patients are by now accustomed to, a better way to fully remove the blue stain from the eyes is something that can relieve patient anxiety and provide comfort for future socialization on the day of ophthalmologist visits.
Week 2: A Clinic Runs Like Clockwork (Storyboarding)
July 5th—July 8th, 2022
This second week of the Clinical Immersion Program was packed with a variety of clinics and subspecialties within the Ophthalmology Department. We had the opportunity to shadow Dr. Ellen Shorter in the Contact Lens Clinic, observe Dr. Van Ann Tran in Oculoplastics performing minor operating room (OR) procedures, and continue to search for potential medical innovations in Dr. Jain’s Dry Eye & oGVHD Clinic. Of all the things we witnessed, my highlight was seeing a dacryocystorhinostomy (surgery for blocked tear ducts involving the insertion of a small plastic tube into the tear duct) and lower eyelid & cheek laceration repair on the same patient. Our eyes remained peeled (quite similar to the patients we saw operated on) to weed out the designs that need improvement, amidst all the other fine-tuned technologies that already exist within ophthalmology.
As we continue to have discussions with Dr. Jain regarding innovation and clinical procedures, we also have noticed how certain elements of the clinic run like clockwork. In an earlier blog post, from Week 1, I mentioned how the very layout of the clinic is streamlined for patient intake, from left to right across the clinic. This contributes to the efficiency, thus clock-likeness, of the clinic itself. An example of one of these clockwork elements is the imaging with the confocal microscope, which typically occurs toward the end of patient intake.
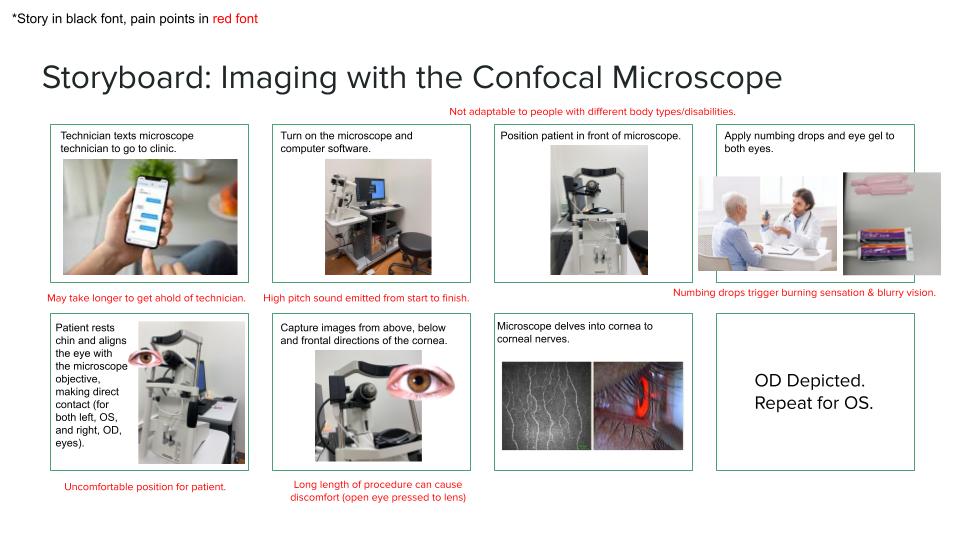
Imaging with the Confocal Microscope
The confocal microscope is a very important tool used in Dr. Jain’s clinic. This technique offers fast and noninvasive in vivo imaging of the cornea, allowing the clinician to track/study corneal nerve alterations over time. In patients with various ocular surface diseases or following corneal surgery, this can be very beneficial in developing the most attuned treatment.
The review paper “In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease,” co-authored by Andrea Cruzat, Yureeda Qazi, and Pedram Hamrah, delves into the importance of confocal microscopy in the field of ophthalmology. The cornea is one of the most densely innervated tissues in the human body, transducing thermal, mechanical, and chemical stimuli as perceptions of pain, as well as maintaining, protecting, and promoting corneal epithelial integrity and, consequently, corneal transparency. Tasked with these important functions, the cornea stands to be a tissue of high interest, thus following the invention of the principle by Goldmann in 1940 and the subsequent patent by Minsky in 1955, confocal microscopy was rapidly applied to the mystery of the eye. The general principle underlying this technique is the alignment of light rays focused on the corneal tissue by the condenser lens, with those reflected by the tissue and captured by the objective lens, hence the term “confocal.” Following many studies and trials of error, confocal microscopy has been perfected to detect causes of corneal neuropathic pain, differentiate between contact-wearers, establish the progression of dry eye disease, and understand ocular dystrophies.
Along with the other members of my group, we created a storyboard documenting the process of imaging with the confocal microscope. A segment of this storyboard is depicted in the image above. Storyboarding consists of mapping out an event step-by-step and calling out potential ‘pain points’ that complicate each step. Whilst most of the pain points describe patient discomfort, we do feel that the benefits of this technique for patient treatment and diagnosis, outweigh the harm.
Citation: Cruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul Surf. 2017 Jan;15(1):15-47. doi: 10.1016/j.jtos.2016.09.004. Epub 2016 Oct 19. PMID: 27771327; PMCID: PMC5512932.
Week 3: Rolling Our Sleeves Up (Need Statement Development)
July 11th—July 15th, 2022
If our immersion was quantified in layers of the cornea (epithelium – Bowman’s layer – stroma – Descemet’s membrane – endothelium), we are now deep in the stroma, pushing past keratocytes and collagen. This week consisted of shadowing clinics and multiple interviews from different subspecialties in the Ophthalmology Department. We learned about inflammatory diseases of the eye (uveitis, iritis, and scleritis) in Dr. Bhat’s clinic, gaped at stories of globe luxation and penetration from Dr. Heinze, and observed the complex machinery (Goldmann and Humphrey) used to conduct visual field tests.
We also participated in a Journal Club with Dr. Jain’s laboratory, where we discussed a paper titled, “Bulbar Redness and Dry Eye Disease: Comparison of a Validated Subjective Grading Scale and an Objective Automated Method.” This paper described the results and conclusions of a prospective study comparing two assessments of ocular (conjunctival) redness—validated bulbar redness (VBR) scale and the Oculus keratograph. The paper concluded that while both instruments were sensitive to changes in ocular redness, the more significant differences between dry eye disease and control participants were found using the VBR scale. This might be to the larger conjunctival area that is visible during subjective assessment via manual adjustment of the eye while peering through the slit lamp microscope. Yet, in our discussion during Journal Club, we found many faults in the conduction of the study, such as interobserver and intraobserver variability with the VBR scale over time, lack of “blinding” of the investigators, missing information regarding the exact technique for handling the keratograph or VBR exams, among other flaws. Ultimately, this discussion prompted our CIP group to start “rolling up our sleeves” and delve into secondary research concerning ocular redness, as we have identified quantifying ocular redness within the eye to be a prime candidate for innovation.
Developing a Need Statement: Residents Suturing over the Ocular Surface
At the start of this week, we were tasked with practicing the development of need statements. Before delving into the problem, I need to set the scene in which I observed the potential for innovation. Last Friday (07/08), our group was in the minor operating room (OR), shadowing several oculoplastic surgeries. The attending on these cases was often assisted by a resident or fellow or even both if the surgery was more complex. The observed problem was the following: A resident was suturing two flaps of the upper eyelid together with a dissolvable, clear thread. While suturing, the resident dropped the needle several times and had a problem finding the thread and drawing the skin together flatly. While this did not influence the quality of the surgical repair, this did waste surgery time, as well as prompted the attending to correct the resident. Thus the questions surfaced; is there a more efficient technique to teach residents how to suture, is there a better method to suture over the ocular surface, and ultimately—is there room for innovation here?
To develop my need statement for this problem, I delved into the POO method. The POO method breaks down into:
P – population (who is your target population?)
O – opportunity (what is the opportunity for innovation?)
O – outcome (what is the primary outcome/deliverable?)
Using this methodology, I was able to apply the neat acronym to the observed problem in minor OR oculoplastic surgeries, as seen above. This acronym plugs into a need statement framework — [Population] has [opportunity] desiring [outcome]. Using the items I identified for POO, my need statement became “Ophthalmology residents struggle with fine, flat suturing, desiring a methodology that will improve time and quality of suturing.” [P – Ophthalmology residents | O – Struggle with fine, flat suturing | O – Improve time and quality of suturing]
Yet after a bit of reflection, I realized that this statement did not encapsulate the problem at hand. Of all the subspecialties within the Ophthalmology Department, only Oculoplastics deal with suturing near/over the orbital surface, thus it would be more accurate to identify oculoplastic residents as my population, and specify the area of suturing. Following these changes, my need statement became “Oculoplastic residents struggle with fine, flat suturing over/near the orbital surface, desiring a methodology that will improve the time and quality of suturing.” [P – Oculoplastic residents | O – Struggle with fine, flat suturing over/near the orbital surface | O – Improve time and quality of suturing]
Re-reading over this second iteration of my need statement, it still seemed like there were some gaps to be addressed. What is the problem I am identifying a need for? The quality of the suturing itself was not impacted by the resident fumbling with the suture thread and needle, as the attending swiftly corrected any mistakes. This narrowed down my problem, as the issue was with time spent to suture, as well as ease/comfort of suturing for the resident. Not to mention the additional pressure in the room, as the surgeries in the minor OR room, and many oculoplastic surgeries, are conducted on conscious patients, with minimal anxiety-relieving medication given to the patient and a local anesthetic applied to the area of operation. Given the complexity of this problem, my final need statement became the following:
Oculoplastic residents struggle with fine, flat suturing over/near the orbital surface on conscious patients, desiring to improve time and ease of suturing. [P – Oculoplastic residents | O – Struggle with fine, flat suturing over/near the orbital surface on conscious patients | O – Improve time and ease of suturing]
This problem can be approached via two paths—from a surgical perspective (ie. the development of a color-changing suturing thread, which changes from black, for ease of suturing by residents, to clear, for the comfort of patient post-surgery) and from a teaching standpoint (ie. the creation of a training seminar/workshop mimicking the motions of a conscious patient and high-stress environment of the minor OR).
Week 4: An Unexpected Argentinian Flag (Patent Search)

July 18th—July 22nd, 2022
Like Ms. Frizzle delving into human bodies with her Magic School Bus (“The Magic School Bus Inside Ralphie”), we continue our deep dive into the wealth of knowledge at the Ophthalmology Department. This week included shadowing Dr. Johnson at the Neuro-Ophthalmology Clinic, where we learned how a neurologist plays into diagnosing matters of the eye, as well as how a physical maneuver (Epley Maneuver) can cure vertigo. Additionally, we followed Dr. Shorter at the Contact Lens Clinic, watching the development of “piggyback lenses,” conducted several interviews across the department, and had the opportunity to shadow cataract surgeries in the major OR (OR 19).
It was in OR 19 where we first heard the reference to the Argentian flag sign. We received directions to arrive at the 3rd floor of the main hospital (1740 W Taylor St, Chicago, IL 60612) at 6:55 AM. Upon arrival, we got scrubbed and entered the hustle and bustle of ongoing surgeries and surgery preparation between the Surgicenter and nineteen OR rooms. Dr. Joltikov was scheduled to conduct five cataract surgeries, with us shadowing her, on her first day as an attending. She was an excellent mentor—pausing after every surgery to check in and see if we had any questions, explaining the types and grading of cataracts, and leading us through the general procedure. Cataract surgery, like many of the surgeries we’ve seen in the Ophthalmology Department, is complicated by the fact that the patient is conscious, with one of their eye pried open using a speculum. To clarify, cataract surgery involves the removal of the lens of the eye and the insertion of an artificial lens, to remove the opacification (cataract) that has formed on the natural lens. Yet, Dr. Joltikov conducted each surgery with grace, reassuring and informing the patient throughout the procedure. The first four cataract surgeries were relatively uncomplicated—straightforward phacoemulsifications, involving three nuclear sclerotic cataracts and one cortical cataract. The fifth and final cataract surgery involved the dreaded white cataract, whereas the cataract is visual as a cloudy circular mass through the cornea, even without a slit lamp microscope. These cataracts are dangerous to operate on due to the lack of visualization, the uncertainty of depth of the cataract, as well as lack of knowledge regarding the actual vision potential to be gained post-operatively. One of the most common operative errors during white cataract surgery is the “Argentinian flag sign,” which arises after a puncture of the anterior capsule encasing the lens. By far, this was one of the most interesting operations I’ve seen, with an undercurrent of tension, and intense relief, when the white cataract turned out to be “fluffy” and easily removed via the phaco. This experience would be one
For our blog post this week, we were tasked with finding one patent relating to a unique need statement. I decided to continue with the need statement (NS) I developed last week: “Oculoplastic residents struggle with fine, flat suturing over/near the orbital surface on conscious patients, desiring to improve time and ease of suturing.”
The patent that I found related to this NS was for a surgical headlight using multiple LEDs, patented by Vikon Surgical, LLC in 2014. [Patent No. US 8,789,962 B2] Vikon Surgical retails this innovation as a “Surgical headlight Vikon® Centurion,” using 12 LEDs for optimal color rendering and better depth perception of the surgical object. This company is known for utilizing unique methods of light to illuminate the surgical field, including the “Surgical headlight Vikon® Featherlight” which uses 300-watt xenon light. The approach consists of a surgical headpiece with at least two light sources supported by the user’s head, a heat sink to dissipate thermal energy from the light sources, a combiner including a scrambler to receive a portion of the light emitted from the light sources, and a lens projecting the substantially-uniform combined light into the surgical field.
The claims of the patent guarantee illumination greater than about 300 lumens, combinations of differently-colored LEDs to deliver varying levels of brightness and power consumption, and other applications of LEDs to portable illumination of the surgical field. This solution begins to approach the need statement I developed last week, a way to assist oculoplastic residents with suturing over/near the ocular surface. Whilst I am not sure this would cut down on the time of suturing, as this may be improved through another methodology, it would make the suturing thread and field more visible, thus increasing the ease of suturing for residents. As I observed the resident suturing in the minor OR during a blepharoplasty the other day (Friday, July 8th), there was an evident problem with the visibility of the clear, dissolvable suturing thread towards the closing of the eyelid. Thus, I do see the benefit of implementation and standardization of a surgical headlamp in the minor OR, where there are less constant/saturated sources of light. In the major OR, where there are enormous, overhead lamps and slit microscopes, and most of the general procedures are more standardized, there are fewer problems with visibility.
Week 5: A Pool of Knowledge (Business Model Construction)
[In full disclosure, this week was dedicated to research and final paper/presentation preparation, as I had a COVID-19 exposure, and soon after tested positive, rendering me to a 10-day quarantine period]
July 25th—July 29th, 2022
With the dawn of the week 5, the month-long immersion begins to set in. We have accumulated countless tidbits of information, from the time spent with Dr. Jain and his technicians at the Dry Eye Clinic to the hours spent shadowing and learning in the clinics/ORs of Dr. Joltikov, Dr. Shorter, Dr. Johnson, Dr. Bhat, and Dr. Tran, to the interviews with Catriona Byrne, Dr. Arteaga, Dr. Heinze, Dr. Yoon, Dr. Katz, and Kiera Byrne. We had approached ophthalmology as a pool, and had jumped right into the 13-foot deep end.
In class we contemplated various business models, looking at general market characteristics for potential competitors and partners, with competing patents, concepts, and products. For the purpose of this blog, I am continuing with the same need statement, restated below:
”Oculoplastic residents struggle with fine, flat suturing over/near the orbital surface on conscious patients, desiring to improve time and ease of suturing.”
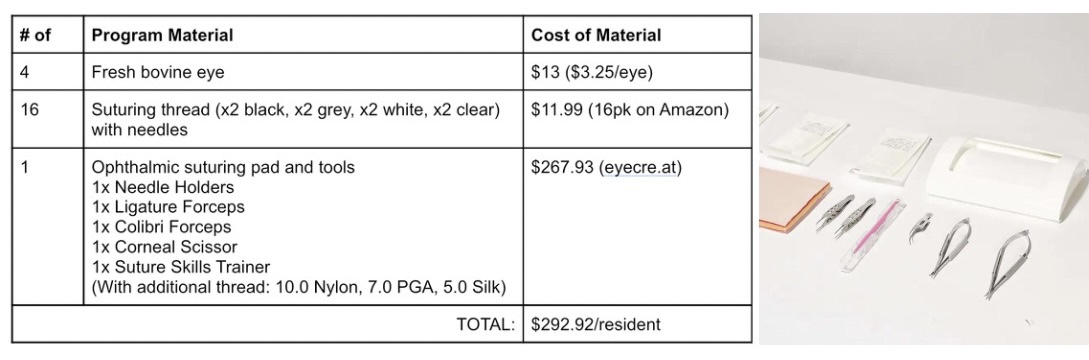
To quantify this particular market segment, I am going to propose the solution of an intense 4-week training program in the “intern year” of the oculoplastics residency. For the purpose of this 4-week training program, the residents will be equipped with a fresh bovine eye each week, suturing threads with a gradually lightening gradient (black to grey to white to clear) with needles, one practice suturing pad, and ophthalmic suturing tools. The cost breakdown per material to estimate potential product cost (solution agnostic) is described in the table above, with the adjacent image depicting the surgery tools.
To understand the number of units (or residents completing the training program), I had to do a bit of research into ophthalmology residencies. Ophthalmology is a very competitive specialty that is further divided into eleven subspecialties, one of which is oculoplastics. According to a fact sheet from the Minority Ophthalmology Mentoring Program (www.aao.org/), 498 residents were accepted into 122 accredited residency programs in the 2020-21 match cycle. Doing some approximation and guess-estimations, 498 residents divided amongst 11 subspecialties leads to 45.27 or ~46 residents in oculoplastics. Thus, providing the training program per year to a pool of 46 residents would cost $13,474.32. The calculations I did above build out my Total Addressable Market (TAM), with the general equation of TAM = # units/year * cost of the product. In the scenario of my need statement, my TAM became: TAM = #residents/year * cost of program materials = $13,474.32. It is important to note that I am not including additional costs of training, such as attending supervision/training, which may insert an hourly pay and/or stipend to the attending for leading the 4-week workshop.
Week 6: With a Cherry On Top (Final Proposal)
This final week of CIP was a hectic, yet organized, push to the finish–gathering resources, numbers, and testimonies to complete our final proposal in presentation and paper format. In completion and reflection of this six-week immersion program, I am in awe of how much I have learned and benefitted from collaboration with my peers and through the guidance of our mentors. In the theme of the numerous analogies that have littered my past blog posts, we have penetrated the final layer of the cornea, the endothelium! We have placed the cherry on top of our banana split sundae! Stick a fork in it; it’s done.
In a brief acknowledgment to everyone who has guided us, I would like to thank Dr. Sandeep Jain, Director of the Dry Eye & oGVHD Clinic, and Christine Mun, Assistant Director of the Dry Eye & oGVHD Clinic, for their time spent introducing us to the field of ophthalmology, answering our questions, and organizing ventures into several subspecialties. Jessica Mun, Annette Garcia, and Bayassa Surenkhuu, Clinical Coordinators at the Dry Eye & oGVHD Clinic, for their patience and willingness to explore patient intake, various ocular examinations, and field our inquiries. Dr. Anthony Felder and Dr. Michael Browne, for their roles in organizing the Clinical Immersion Program and providing a foundation upon which to build medical innovation. I am also incredibly grateful to my group members, Neil Sundaram and Liz Troy, rising M2 students, and Jennifer Meza, a fellow rising BME student. It has been a pleasure to get to know them and work together to discover room for medical innovation within the Opthalmology Department, and I hope our paths will cross in the future.
For future participants of the CIP program, I advise them to enter each department with an open mind—without bias, delusion, or expectations. Medical innovation can be as small as replacing a taped instrument with a customized grip, and yet have a major impact on treatment or procedural delivery. However, to see such medical innovations, you need to think outside of the constraints of traditional classroom and textbook knowledge. I challenge future participants to think bigger and be better! Good luck!