
Jennifer Meza
Student Participant
Ophthalmology
Week 1: Good and Bad Designs
Week of: June 28, 2022 – July 1, 2022
The first week of the Clinical Immersion Program was a rewarding one for sure. My team and I shadowed Dr. Jain as well as three other lab technicians, Annette, Christine and Jessica in the department of ophthalmology at UI Health. More specifically, Dr. Jain is the director of the Dry Eye and Ocular GVHD Clinic where he sees approximately 17-20 patients a day. The AEIOU framework helped guide our observations and focus toward activities, environments, interactions, objects, and users. Through the lens of this framework, our team sought to identify good and bad designs in the clinic.
During our first few days there, we were able to observe a typical exam workup with several patients having different diagnoses. New patient workups were much more extensive at approximately two hours whereas revisiting patients could take anywhere from ten to twenty minutes. This lapse in time is due mostly because new patients have to undergo several preliminary exams and must give extensive background history to have a proper starting point for a diagnosis.
Typically, preliminary exams and patient history is gathered for new patients by one the lab technicians while Dr. Jain mainly views patients under the slit lamp microscope while performing different stainings.
One of the good designs my team and I noted was the layout and flow of the environment of the exam rooms and clinic in general.
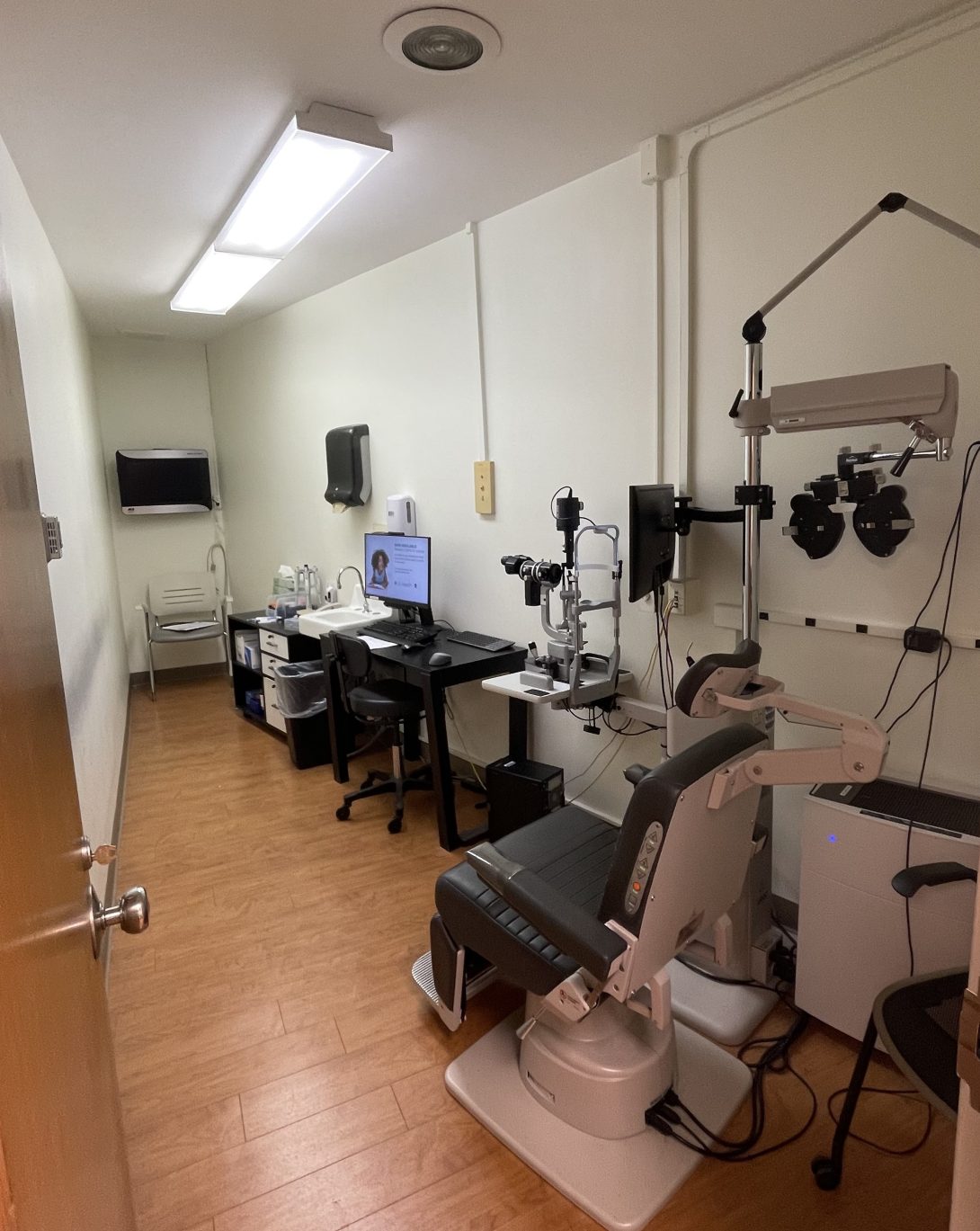
Exam rooms had all furniture and equipment on one side of the room, both optimizing space and allowing for a walkway so both the doctor and technician could pass through. As for the clinic itself, upon entry, there is a purposeful flow to the different rooms. From left to right, there are two machine rooms containing the various equipment such as the keratograph, then follow the two patient exam rooms, and finally, right outside the last patient exam room, to the very right, is the confocal microscope.
The flow of a typical patient exam with Dr. Jain and one other technician further solidified the streamline set up of the exam room. The modification of having only one technician in the room with Dr. Jain was a result of a higher patient volume that is being acquired. However, my team observed that having one technician was sufficient for eye staining and aiding in the process of tear production.
As shown in the photo above, the table containing pipettes and other materials used for staining and tear collection is furthest away from the patient and the computer is right next to the patient. While I had initially thought the two components should be switched, I quickly realized this may not be the best solution. Dr. Jain must access the computer readily and is usually seated directly in front of the patient from viewing the eye using the slit lamp microscope which makes the transition from patient to computer smoother than if he had to travel across the room. Overall, Dr. Jain and each of the technicians have a mutual rhythm when it comes to performing staining and tear collection. Technicians know what Dr. Jain needs and when, sometimes even without his asking.
Some other good and bad designs that my team and I observed are as follows:
Good: devices used for new patient workups (i.e. keratograph, optometer) for the most part have computer screens that rotate so patients can see images as the results are explained by a technician, use of micropipettes which administers a precise volume of lissamine green staining
Bad: wheelchairs cannot fit into the narrow doorways of exam rooms, eye washing is frequent and the dye can leave some staining around the eyes, keratograph does not capture the entirety of the conjunctiva (whites of the eye) and sometimes captures part of the eyelids which results in an inaccurate quantification of ocular redness.
In general, this week was full of several observations and the establishment of some preliminary needs. However, as a team, we learned that whether it is a good or bad design, both are susceptible to innovation and innovation without impact is meaningless. Going forward, the goal is to keep this in mind when identifying needs in the clinical setting.
Week 2: Storyboard

During the course of this week, my team and I were given the opportunity to work in three different clinics at UI Health. Aside from our “home base” at Dr. Jain’s Dry Eye and GVHD clinic, we were privileged enough to observe and shadow in the contact lens clinic as well as in oculoplastics. Given our familiarity with Dr. Jain’s clinic, I decided to storyboard corneal confocal microscopy that takes place in his clinic to identify the relevant steps and pain points associated with the process.
In the paper co-authored by Yukihiro Matsumoto and Osama M. A. Ibrahim titled, “Application of In Vivo Confocal Microscopy in Dry Eye Disease,” there is a strong emphasis on the multifaceted opportunities that the confocal microscope provides in the identification of dry eye disease. Currently, in Dr. Jain’s clinic, our team has observed the use of confocal microscopy on the cornea to identify inflammatory cells or areas on the epithelium as well as some of its sublayers. However, this paper summarizes the use of confocal microscopy as a noninvasive procedure for assessing treatment effectiveness. Specifically, the paper emphasizes the versatility of such a test to be used to determine treatment effectiveness presented in the morphologies of the conjunctiva and Meibomian glands, for example.
From interviews and discussions with Dr. Jain, a major part of determining the extent of dry eye lies in inflammation which can attribute to the presence or absence of redness. One of the major challenges is in quantifying ocular redness in a consistent manner. The peer reviewed paper mentioned above brings attention to the many functions of a confocal microscope and how it characterizes the presence of inflammation in the various morphological aspects of different structures. There seems to be a divide between inflammation being characterized by confocal microscopy and the quantification of ocular redness, but what if they could be bridged through the standardization of the confocal microscope? The paper aforementioned indicates that the confocal microscope still requires higher levels of sensitivity and specificity when it comes to structures of the eyes, however, maybe it is a starting point for understanding how to quantify or standardize the relationship between inflammation and redness in ocular pathologies.
Some more detail on my experience in the contact lens clinic and in oculoplastics…
On Wednesday, my team and I met with Dr. Ellen Shorter in the contact lens clinic which goes beyond the typical preference for contact lenses. Her clinic specializes in providing contact lenses for people that have experienced some sort of trauma to the eye making regular contact lenses uncomfortable and irritating to the eyes. Oftentimes, her patients are referred to her by Dr. Jain due to dry eye or GVHD discomfort.
On Friday, my team and I observed minor OR procedures in Dr. Van Ann Tran’s clinic of oculoplastics. We were able to observe two blepharoplasty procedures which remove excess skin from the eyelids and is used for patients with droopy eyelids. One patient expressed gratitude to the surgeon, resident, fellow and even toward our CIP team after the procedure. Dr. Tran immediately showed the patient a picture of the eyes and the patient was so happy with the results. It was such a rewarding experience to see how Dr. Tran’s work made such an impact on the patient as well as to see the patient’s gratitude toward everyone there to support her and help her while in the minor OR despite a language barrier as well. I look forward to witnessing other patient testimonies on the impacts that ophthalmology doctors have had on their lives.
Citations:
Matsumoto, Yukihiro, and Osama M. A. Ibrahim. “Application of In Vivo Confocal Microscopy in Dry Eye Disease.” Investigative Ophthalmology & Visual Science., vol. 59, no. 14, 2018, pp. DES41–DES47, https://doi.org/10.1167/iovs.17-23602.
Week 3: Needs Statement Development
This week in the Ophthalmology Department, my team and I had the pleasure of interviewing several staff members in different subspecialties. We learned a lot about their experiences as individuals working in a clinical setting and were able to hone in on some of the problems each subspecialty encounters to get a better basis for underlying needs and the potential for innovative solutions. We shadowed Dr. Pooja Bhat who mainly sees patients with uveitis, talked to Dr. Kevin Heinze, an oculoplastics fellow who gave insight on some potential areas for innovation and even discussed very extreme cases he had encountered such as penetrated eyes resulting in a globe surgery procedure. During an interview with a neuro ophthalmology technician, Kiera Byrne, showed us two visual field machines, the Goldmann and the Humphrey. Something that stood out to me was Kiera’s description of her interactions with patients and how they have often suffered a great deal and have tragic stories behind their ailments. She mentioned something that patients frequently comment: “Don’t take your vision for granted.”
Another focus for this week was to practice developing needs statements. To tackle this, we were given a useful framework:
P – Population (Who is the targeted population?)
O – Opportunity (What is the challenge or problem at hand?)
O – Outcome (What is the result of addressing the opportunity? i.e. validation)
Using this format, I was able to come up with three different iterations of a needs statement pertaining to surgical trays used in the oculoplastics minor operating room (OR). To give some background on what a minor OR looks like in oculoplastics, patients are undergoing a wide variety of procedures that involve the orbit, eyelids, tear ducts and/or face while involving reconstruction of the eye as necessary. These ocular surgeries require eye drops to protect the inner eye and topical anesthesia in the form of a syringe to numb the outside and surrounding areas of the eye. Patients are awake and alert and do not require general anesthesia.
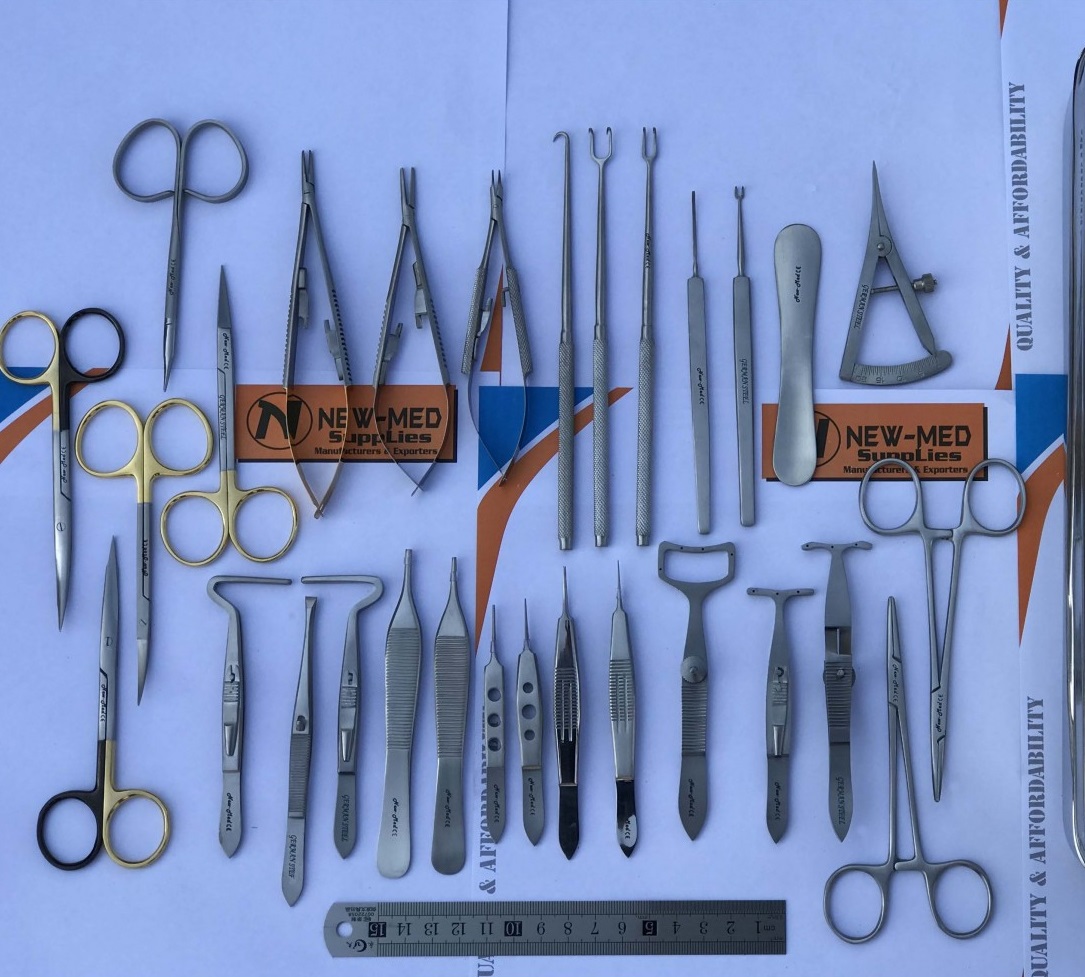
As in any OR, sterilized surgical tools are prepped for use. My team and I observed this process as the scrub nurse opened each tool from its sealed bag and simply dropped the tools on the surgical tray one at a time. The tools were not rearranged in any particular way and the surgeon, Dr. Tran, proceeded to begin the first incision by reaching for the scalpel herself. In this particular instance, she was joined by a resident doctor and a fellow as well. As the procedure progressed, Dr. Tran reached for each tool when necessary and was able to find each tool fairly easily. As for the resident and fellow, this process of looking for the right tool was a bit more lengthy.
This evoked some thoughts about how a surgical tray that is not organized in a systematic way can be a problem if residents and fellows struggle to find a particular tool and how looking for the tool can result in noise that could make patients more uneasy during an already awake procedure. I identified the following components using the “POO” framework to construct a viable needs statement:
Population: oculoplastic surgeons operating
Opportunity: systematic organization of tools on operating tray for optimal handling
Outcome: shorter procedure time
This resulted in the following needs statement:
Oculoplastic surgeons operating on a patient need a systematic layout of surgical tools on the operating tray so they can access the tools efficiently, resulting in a shorter procedure time.
However, given that the lack of organized surgical tools largely affects residents and/or fellows, as gathered during my observations, I thought the population could be better adjusted to specify further.
Oculoplastic residents and/or fellows operating on a patient need a systematic layout of surgical tools on the operating tray for better handling and a shorter procedure time.
After careful consideration of what a desirable outcome could entail, I decided that perhaps this organization of surgical tools could also be beneficial for standardizing the tools used for each procedure. Something I noticed when observing in the Department of Oculoplastics was that sometimes Dr. Tran needed more sutures or a different tool that was not initially included on the tray. She needed to call the nurse to come bring the tool and sometimes the nurse could not hear her because she was in the next room, prepping it for the next procedure. This lengthy process results in a longer procedure time and could result in more topical anesthesia being administered to the patient if it “wears off.” To account for some of these problems, I decided to further modify my needs statement:
Oculoplastic surgeons operating on a patient need a systematic layout of surgical tools on the operating tray to account for excess tools and materials according to the specifics of the procedure to minimize any noise that can make conscious patients uneasy and ultimately result in a shorter procedure time.
Week 4: Patients and Patents
Having interviewed several doctors, technicians and medical students across the subspecialties in the Department of Ophthalmology, my team and I have identified a common trend. At some point in each interview, we prompt the interviewee with the following question: “What are some areas of improvement or any pain points associated within your clinic?” To which several interviewees have responded to with scheduling issues. Scheduling issues refers to a whole slew of underlying process issues within the clinic such as the lack of a streamline pathway for patient imaging which then increases patient visit time quite a bit. It can also involve the major staffing issue common in the General Eye Clinic (GEC) where attendings are required to sign off on residents which is a practice that can back up the flow and efficiency of the clinic. In an effort to frame the question differently, we usually try to direct interviewees back to their use of instrumentation and their interactions with it to try and identify pain points. This usually prompts a long moment of pondering … followed by “I’m not sure” or “It’s always been that way.”
Physicians employ workarounds without even realizing it. All. The. Time. For example, when using a Pentacam which is used for corneal imaging, alcohol pad packets were placed between the patient’s forehead and the rubber band fixing their head in place to ensure more optimal positioning when switching between each of the four quadrant scans of the eye (nasal, temporal, superior and inferior). Other times, it is simply the notion of “It’s always been that way” that prompts some physicians to overlook areas of improvement. Patients from the dry eye clinic are used to walking out with Avatar blue stained skin from the lissamine green dye and technicians do the best they can to remove most of it but it has always been that patients often leave with at least some staining surrounding the eye.
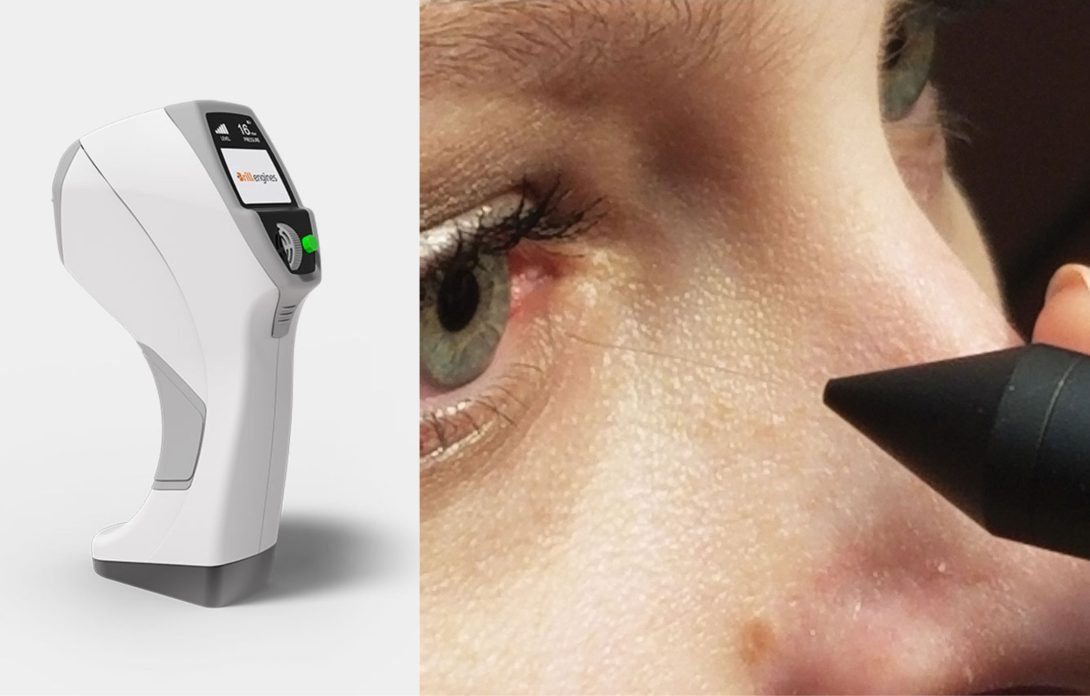
Corneal Aesthesiometer Brill by Brill Engines
After brainstorming several need statements rooted in our observations from the past few weeks, my team and I have looked through a lens of innovation where we question nearly every process or use of a device which makes for good breeding soil for need statements. However, what about other devices or methods that are “out there” which can possibly address these needs? I’ve decided to focus on the following need statement:
Dry Eye Disease (DED) patients struggle with discomfort from the corneal esthesiometer pen and require a non-contact device to measure corneal sensitivity.
The current method used in my primary observation in Dr. Jain’s clinic is a pen which extends a fine nylon filament that touches the patient’s corneal surface. The longer the filament, the greater its flexibility, indicating a greater degree of corneal sensation if the patient’s blink reflex is present. An illustration of this device is shown in the image above, on the right-hand side.
A 2019 invention measures corneal sensitivity by employing puffs of air at different intensities to measure tactile sensation of the cornea. The Corneal Esthesiometer Brill by Brill Engines is a novel device that claims to be the “first portable, non-invasive, accurate and precise esthesiometer available.” The aesthesiometer contains a source of pressurized gas, an expandable cavity which is used to contain a specific volume of the gas. Aside from this, the device contains valves to direct and administer the gas at a constant pressure.
Relevant Claims
One of the claims outlined in the patent emphasizes the release of the puff of the volume of gas to be released in a controlled manner from the expandable cavity to make sure that the pressurized gas remains “a substantially constant outlet pressure.” Another claim describes the mechanism of the expandable cavity comprising elastic potential energy accumulation during the loading phase of the device so that the “said potential energy in the firing phase” is the same. A majority of the claims outline the physics of the device such as the mechanics of the constant-force spring and elastic potential energy accumulation.
Although this device offers a potential improvement to the existing method of the nylon filament alternative, it also introduces a large degree of subjectivity to the test results. This non-contact device requires patients to verbally confirm whether they felt the puff of air which reduces the integrity of the test. As mentioned above, testing corneal sensation can be very telling as to whether patients are experiencing neurotrophic keratitis which indicates corneal degeneration causing loss of sensitivity and can result in several ocular pathologies such as ulcers, aseptic necrosis and perforation of the cornea. Given that this exam can be very informative, perhaps using the traditional nylon filament which causes patient discomfort for a few seconds at a time would be better in order to ensure the integrity of the results. Furthermore, the puff of air may introduce subjectivity in that patients could report sensation that is not due to the eyes but rather the surrounding skin area. In either case, it is likely better for patients to undergo some momentary discomfort rather than introduce subjectivity to the exam.
Week 5: Addressing the Market...
The past few weeks have culminated in a slew of clinical needs ranging from a desire to better clean off lissamine green staining from patient eyes to needing a better way to quantify ocular redness that reduces subjectivity of grading scales and increases accuracy of the full conjunctival field. When exploring the corresponding problem spaces, it became apparent that calculating a total addressable market is a different story for each case. To get a better understanding of this, I’ll define the total addressable market as outlined in our program didactics:
Total addressable market (TAM): overall revenue opportunity that is available to a product or service is 100% market share was achieved
Two things are needed to calculate the TAM, the first being the number of units per year and the second being the cost of the product. Then, TAM is calculated by multiplying the two numbers together. While this calculation is very simple, my team realized that there are several different routes to approach what the number of units per year would encompass. The most straightforward way is to use a given number of patients if the product is “one per patient” such as eye patches that could be placed underneath the eyes of dry eye patients who undergo lissamine green staining. This number could be easily calculated using the number of dry eye patients per year while considering the number of visits each patient has per year. On the other hand, considering a product to measure ocular redness such as some form of handheld spectrometer device to be used by clinicians, introduces a more complicated market. Not only is there limited data on the number of ophthalmology clinics in the U.S. but the existing data is outdated. Another factor to consider is the number of clinicians in each relevant clinic. Considering ocular redness can be seen across ophthalmic subspecialties, the market needs to clearly cater to the appropriate clinics to get a better sense of the number of clinicians that would be utilizing the product. This route to calculating TAM is clearly very cumbersome and introduces several layers of specificity.
To better understand the basis for our problem space, I’ve included our needs statement below, outlining the “POO” method:
Ophthalmologists struggle with measuring bulbar redness as a proxy for inflammation and desire a precise methodology to quantify progression of ocular disease and better titrate patient treatment.
P – Population: ophthalmologists/ophthalmology technicians
O – Opportunity: improved methodology for quantifying ocular redness
O – Outcome: better treatment titration
A more accurate scope of the TAM would utilize the number of uses of a handheld spectrometer instead of the traditional, “#units/year.” To approach this problem, the specific market segment for the use of handheld spectrometer devices that measure ocular redness would be approximately 16.4 million patients [1]. This number corresponds to the average number of patients with dry eye disease annually. Assuming patients visit an ophthalmology clinic every 1-6 months and utilize the handheld spectrometer device each time, the market segment increases as follows: 16.4 million x (1-6 mo.) = 32.8 million – 114.8 million office visits per year.
This would result in the following breakdown:
# uses per year = 32.8 million – 114.8 million
Cost of product = cost of external photography exam on Oculus Keratograph 5M = $23.38 [2].
TAM = (32.8 million-114.8 million) x $23.38 = ~ $786 million to ~ 2 billion
Citations:
[1] Kimberly F. Farrand, Moshe Fridman, Ipek Özer Stillman, Debra A. Schaumberg, Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older, American Journal of Ophthalmology, vol 182, 2017, pp. 90-98, ISSN 0002-9394, https://doi.org/10.1016/j.ajo.2017.06.033.
[2] “Corcoran Consulting: Ophthalmology and Optometry Practice Management.” Corcoran Consulting Group, Corcoran Consulting Group, 28 Sept. 2020, https://www.corcoranccg.com/.
Week 6: The End of the Beginning

In reflecting on the past six weeks of the Clinical Immersion Program, I realize that it has brought clarity to my future career path as well as a slew of skills and knowledge that I anticipate will be of great advantage as I pursue a position in the medical device industry. I am so grateful for the educators, Dr. Anthony Felder, Dr. Michael Browne and Dr. Miri Kotche, who have poured their time and efforts into this prestigious program.
It was an amazing experience to work with my team, Julie Gawenda, Neil Sundaram, and Liz Troy in the Ophthalmology Department. We learned so much together and I learned a lot from each of them as individuals as well. This internship experience highlighted the missing pieces in my engineering background and knowledge of medical devices and subsequently filled in the gaps. From BIOE 101 my freshman year where CIP was first introduced to students by Dr. Felder, to being one of ten biomedical engineering students chosen to participate, I could not have asked for a more fulfilling experience.
I look forward to highlighting this experience for future employers and hopefully seeing our team’s project on “Refining Objective Measurement of Bulbar Conjunctival Redness,” be further developed in our Senior Design Course this coming Fall 2022.
Advice to Future CIP Participants:
- Be confident in your abilities but also ask questions even when you think you might know vaguely how something works. Several ideas for clinical needs came up during discussion with our clinical mentor, Dr. Jain and he helped our team try to think outside the box in terms of medical innovation. Thinking outside the box is challenging but having conversations with your mentor and interviewing others in the clinical setting is very beneficial.
- Try to remember your role is to identify and validate medical needs and not to create a solution. This is really hard and can be difficult for mentors and others working in the clinical setting to grasp but try to immerse yourself in all there is to know and accomplish by identifying and validating the needs. There is more to it than it seems.
- Use your time wisely. Six weeks can seem like a long time, but it is crucial to get the most out of each day and experience in the clinic. Taking the time to reflect on these experiences can put a need statement into perspective and help with its refinement.
Acknowledgements:
Thank you to Dr. Anthony Felder, Dr. Michael Browne and Dr. Miri Kotche for their efforts in facilitating this program and offering guidance all throughout to help our team be successful. To the UI Health Department of Ophthalmology, for allowing students to observe and interact with the staff and equipment. To our clinical mentor, Dr. Sandeep Jain, Director of the Dry Eye & oGVHD Clinic, and Assistant Director Christine Mun for the mentorship and willingness to schedule time in various ophthalmology subspecialties. To Jessica Mun, Annette Garcia, and Bayassa Surenkhuu, Clinical Coordinators in the clinic for their willingness to answer questions and offer their expertise. Finally, a huge thank you to my team, Neil Sundaram and Liz Troy, rising M2 students and Julie Gawenda, a fellow BME student for their collaboration and enthusiasm in exploring this project.
About
I am so excited to be part of the Clinical Immersion Program after its long intermission during the pandemic. Through this program I hope to gain insight on the process of medical device development and how transformative and incremental innovation can be solicited to make impactful changes in the clinical setting. I am currently entering my 4th year as an undergraduate in biomedical engineering interested in pursuing a career in biotechnology within the industry upon graduation.
Year: Senior Biomedical Engineering Undergraduate
Area of Research: Biotechnology
Department: Ophthalmology with Dr. Jain, Director of the Dry Eye & Ocular GVHD Clinic at UI Health
Contact Information: jmeza9@uic.edu