
Jonathan Silberstein
Medical Student
Urology
Email:
Week 1 - Design Winners and Losers
Good design:
Flexible disposable Cystoscope (Ambu 4)
Activity:
The most frequent procedure we saw, thought to be the bread and butter of Urology, was the in-clinic cystoscopy. This procedure is (usually) performed quickly (under 5 minutes), and thoroughly with the patient fully awake. It was often done with one other assistant.
Environment
Cystoscopies can be done in outpatient clinic (more diagnostic) or as an outpatient surgery (usually associated with some form of treatment). In the clinic, we noted one monitor that was connected to the device, and there was very little set-up needed to get a clear image.
Interactions
The cystoscopy is driven entirely by the physician, with the cystoscope in his/her right hand and the free hand holding the genitals to securely control. What I found to be simple and elegant is the way in which the device is maneuvered and controlled with a single hand. This got complicated when there were accessory devices used (such as grippers, graspers, baskets, or biopsy). I noticed the doctor controlling all aspects of the scope by moving his wrist ini conjunction with sliding the latch with his thumb which controls the flex/angle of the camera tip. There was also the interaction of the nurse and the doctor, coordinating the events in the room.
Objects
To begin, antiseptic meds are used to clean the genitals, a numbing lidocaine gel is used to numb the urethra and bladder, and then saline is almost constantly being infused through the scope. The star of the show is the sterilized, single use flexible cystoscope, which had very little setup time, and worked excellently to visualize the bladder and urethra, with a high quality camera.
Users
Physician – each doctor has different preferences for navigating with the scope, but ultimately the steps were the same and the controls seem straightforward and intuitive, even to a non-user
Assistant/Nurse – Communication is key, but seemingly not quite as crucial when there was only one operator of the device.
Bad design:
Wire/Accessory management
Activity
During surgical cystoscopy/ureteroscopy, there were many different accessory components that were threaded down the scope to be used during the procedure. Looking at the sterile table, there were numerous wires and peripherals, all coiled or strewn on the table. These would then be handed to the surgeon when needed and handed back and swapped.
Environment
Operating Room with a sterile table of instruments located just behind the surgeon
Interactions
The scrub nurse organizing and handing/receiving devices to/from the surgeon. Devices needed to stay sterile and ready to be redeployed
The surgeon communicating his needs to the nurse, and then manipulating the tiny wires (often with difficulty). Once, a device was dropped out of the sterile field and a new, sterile device had to be brought in.
Objects
Guidewires, cystoscope accessories (grippers, basket, laser), sheaths, stents
Users
Resident Surgeon, attending surgeon, scrub nurse, surgical tech
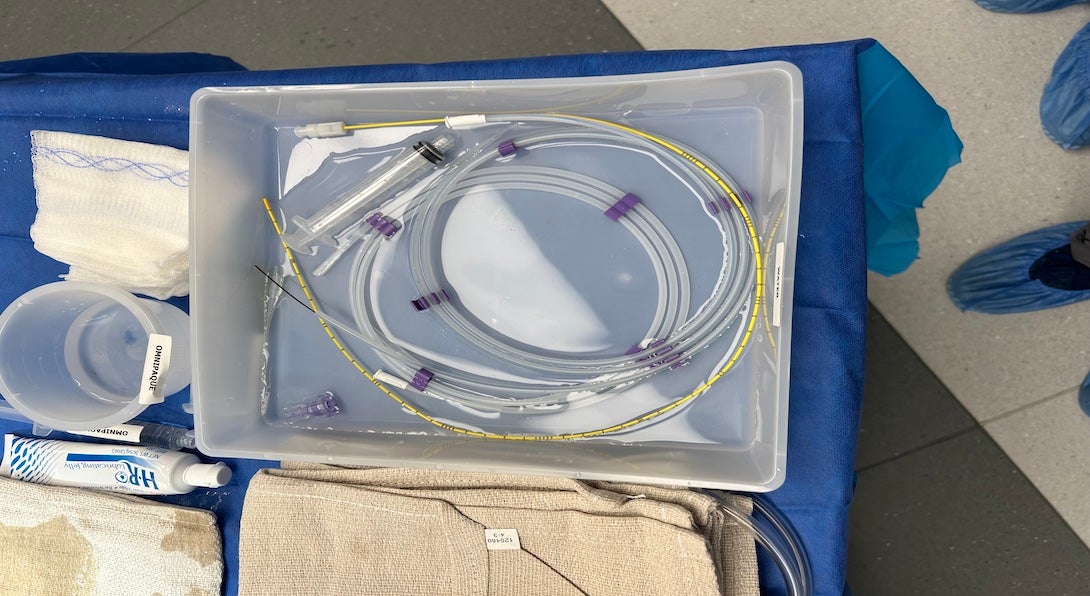
Week 2 - De-stoning: What does the lit say?
Over the past couple weeks, I have had the opportunity to shadow Urologic procedures involving ureteroscopy, cystoscopy and laser lithotripsy stone ablation. A common series of observations I had made involved visualization and the complications that arose with seeing accessory devices and the foreign bodies.
Summary of Journal Article
To this end, I found a peer reviewed article looking at the complication rates and severity of incomplete removal of kidney stone removal. A goal of mine when reviewing articles was to understand the negative impacts that incomplete stone removal can have on a patient, and on the healthcare system.
The primary outcome of the paper I have identified was the proportion of stone events after the initial laser treatment. Stone events included stone growth, stone passage, re-intervention, or post-op complications. The study followed 232 patients across 6 centers who had documented kidney stone fragments after ureteroscopy. Shockingly, 44% of all patients with fragments had a stone event, with 29% of all patients undergoing re-intervention. There were significantly higher complication rates when the fragments were identified to be >4mm, compared to patients with stones <4mm in size.
There is clearly a large cost to the patient, and as a result, the healthcare system due to incomplete clearance of kidney stones during ureteroscopy. This review inspired questions surrounding the root cause of incomplete clearance and methodologies for verifying any leftover stone fragments. I intend to continue to follow up on my research (both primary and secondary) to dig deeper into these questions guide my inquiries.
Chew, B. H., Brotherhood, H. L., Sur, R. L., Wang, A. Q., Knudsen, B. E., Yong, C., … Humphreys, M. R. (2016). Natural History, Complications and Re-Intervention Rates of Asymptomatic Residual Stone Fragments after Ureteroscopy: a Report from the EDGE Research Consortium. Journal of Urology, 195(4 Part 1), 982–986. https://doi.org/10.1016/j.juro.2015.11.009 (Original work published April 1, 2016)
Summary of Patent Review
Laser Lithotripsy Device Using Suction – JP4310049B2
In trying to better understand what others have already done to address some opportunities from our observations, I was interested in finding patents for methods to clear debris in the field of view during laser lithotripsy. This patent for a ureteric suction catheter is assigned to Boston Scientific Ltd. The main claims of this patent are for the inclusion of a suction channel to a device used in lithotripsy treatment with other channels for stone fragmentation and camera visualization. There were included claims around ensuring energy is properly channeled and distributed for stone fragmentation, and for preventing clogging of the suction port. It was originally filled on 2/18 in 2000, and the patent recently expired on 2/18 in 2020. My interpretation of the motivation behind their patent is to make the process of stone fragmentation more efficient by making it easier to eliminate fragments, and also to prevent buildup of debris in the calyces to make visualization and navigation easier. While skimming through the patent, I found the drawings to be very simplistic but numerous to cover a lot of different embodiments. The claims are also fairly broad, and use very generic language, making sure not to specify exact designs but rather allow for many designs to fall under the same claim.
Week 3 - Got a Need to (not have) Pee'd
Needs Statement – First Pass
Patients receiving ureteral stents experiencing disproportionate levels of discomfort and incontinence/freq. desire an improvement in bladder score/QoL
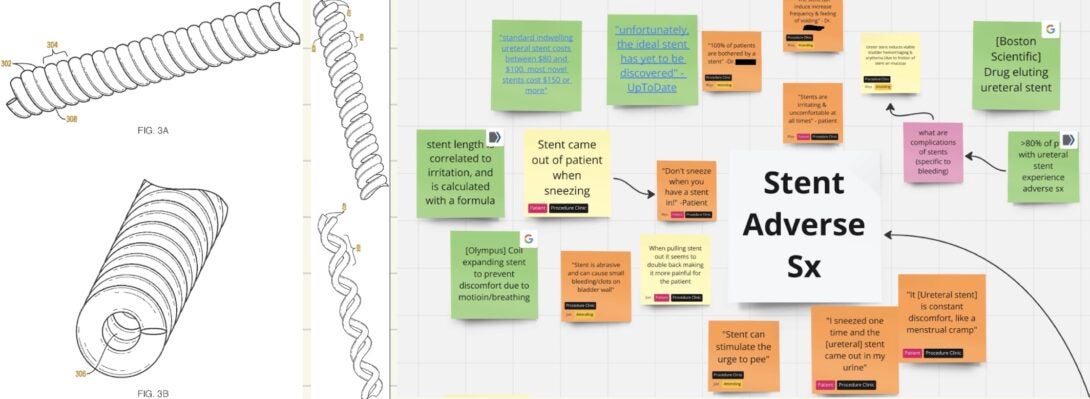
Much of my experience thus far, in seeking opportunities for change, has centered around understanding the physician and their team. This need was different – patients couldn’t wait to tell us about how annoying the ureteral stents they had in were. They couldn’t come out soon enough, even through the discomfort of the procedure. We heard mentions of frequent urges to use the bathroom, laughing or sneezing and immediately regretting it due to the pain, and a constant, unmistakable discomfort. Doctors corroborated this by sharing that “practically 100% of patients [with ureteral stents] experience discomfort”, and the literature says stent related symptoms affect over 80% of patients1. Patients were begging for an alternative solution, and doctors could only empathize.
Preliminary research made it clear that we were not the only ones interested in this opportunity. There are dozens of patents covering coatings, materials, tip design, and even alternative stent stylings, but very few of them on the market seem to actually make a significant difference. One design I found to be particularly novel and clever was the expandable coil lumen stent (see image above), patent US11213414B2 held by Boston Scientific2.
The Ureteral stent market has a handful of major players, including Boston Scientific, Coloplast, Teleflex, and Olympus to name a few. It is a somewhat fragmented market, and companies are constantly working on developing more comfortable, safer stents3. This indicates to me that companies are hungry for a competitive edge, and a good solution to this problem will be incredibly valuable.
Total Addressable Market (TAM)
92,000 stents/yr * 80% of stents placed * $840/yr to alleviate symptoms = $61.82 MM USD / yr
92,000 ureteral stents placed in the US/yr5
~$500 average yearly costs of incontinence4
$840/yr amount women were willing to pay for resolution of incontinence4
80% of patients experiencing stent symptoms1
P – Patients who have ureteral stents and experience stent symptoms
O – Relieving symptoms of discomfort/urinary urge caused by stent
O – Increased QoL or decreased stent symptoms
Needs Statement – Do it again, but better
Patients with ureteral stents that experience stent related urge/incontinence need a stent which decreases the incidence/severity of incontinence as measured by the Urogenital Distress Inventory-6 (UDI)6
Rationale: To further improve my initial attempt at a need statement, I wanted to hone in on a specific stent side effect. I chose urge/incontinence, because I have seen in the clinic and through literature how much of a negative impact this specific symptom has on people’s lives. It also is one of the most common stent symptoms, so it felt like an important one to focus on. Finally, I wanted to include a quantitative way of measuring success. There are many validated questionnaires that help diagnose severity and QoL impacts of incontinence, so I specified one that was Grade A recommended and used for both men and women.
Citations:
1Miyaoka R, Monga M. Ureteral stent discomfort: Etiology and management. Indian J Urol. 2009;25(4):455-460. doi:10.4103/0970-1591.57910
2US11213414B2, Controlled Extension Stent, US, 2022, Kimberly DeGraaf (Boston Scientific)
3Ureteral Stents Market Size & Share, Growth Analysis Report 2032. Global Market Insights. Published 2023. Accessed July 13, 2024. https://www.gminsights.com/industry-analysis/ureteral-stents-market
4Subak LL, Brown JS, Kraus SR, et al. The “costs” of urinary incontinence for women. Obstet Gynecol. 2006;107(4):908-916. doi:10.1097/01.AOG.0000206213.48334.09
5Gadzhiev, N., Gorelov, D., Malkhasyan, V. et al. Comparison of silicone versus polyurethane ureteral stents: a prospective controlled study. BMC Urol 20, 10 (2020). https://doi.org/10.1186/s12894-020-0577-y
6Naughton MJ, Donovan J, Badia X, et al. Symptom severity and QOL scales for urinary incontinence. Gastroenterology. 2004;126(1 Suppl 1):S114-S123. doi:10.1053/j.gastro.2003.10.059
Week 4 - If We Build It, They Will Come. But Can We Build It?
I ended last week feeling decent about the need I had crafted:
Prior Need: Patients with ureteral stents that experience stent-related urge/incontinence need a stent which decreases the incidence/severity of incontinence as measured by the Urogenital Distress Inventory-6 (UDI).
After reassessing the three (3) pillars of the IDEO model, I wanted to revisit that need and pull in components from each.
Desirability (D) – Through weeks of talking with patients, physicians and conducting thorough literature reviews, I am convinced that this need addresses a significant desire. Read my week 3 blog post, where I go in-depth about the desire and opportunity for a better solution.
Feasibility (F) – My biggest concerns while exploring this need were the resources, knowledge, and time needed to redesign and test a stent. The companies working on this problem have essentially limitless financial resources, and teams of PhDs who have dedicated their entire careers to materials sciences, and they still haven’t come up with great solutions. As an idea to expand the problem scope, I decided to remove the stent as the solution from the need. I tried to shape the language so as not to inherently limit my need to fixing the current standard (which is a stent), but rather leave it open to the possibility that a stent may not be the best solution.
Viability (V) – Ensuring adequate drainage of the kidneys is a crucial step after nearly every procedure where the ureters are traversed or disturbed, or when there is a pre-existing blockage; the ureteral stent is the go-to tool in the Urologists toolkit for that exact purpose. According to one of our physician mentors, before stents were common there was the nephrostomy, or a direct drain from the kidney to an external collecting bag through the back. There will always be a need to maintain patent ureters, and whatever solution relieves patients of added urinary symptoms and functions well will have a reserved spot in the Urologists work flow. Viability will depend on the ability of the solution to be easy to implement for the physician, and less miserable for the patient.
Patients with ureteral stents that experience stent-related urge/incontinence need an easy to employ(V) method to provide adequate drainage of the kidneys(F) which decreases the incidence or severity of incontinence(D,V) as measured by the Urogenital Distress Inventory-6 (UDI).
To make sure we’re keeping our eyes open, and not getting locked into one need too early, I have addressed another opportunity that I’ve noticed longitudinally in the OR and Procedure rooms. This stems from having witnessed so many leaks and missed connections of fluid lines. The connectors are ubiquitously Luer Lock, which require screwing one device onto another, and are often very small and easy to miss. The outcome is fluid leaking on the patient, the drapes, the floor, making the environment more dangerous, while wasting both time and precious fluids.
HC Workers operating in the sterile field need a method to quickly and reliably attach fluid lines with one hand to decrease the volume of fluids spilled during a case.