
Izabela Stankiewicz
Medical Student
Email:
Week 1: AEIOU and, sometimes, "why?"

QOTW: “There are errors and then there are critical errors.” – [overheard in OR 11]
Good Design – W&H Implantmed Drill
Activity
In one of the most collaborative surgeries witnessed during the week, we got to observe how a prosthodontist was able to instal 12 dental implants in a patient with minimal structure left to their maxilla and mandible. This drill (set-up pictured on the far right of the main image) was necessary for both boring holes into the jaw facial bones and actual placement of double-threaded implants into these tunnels. The drill was connected to a bag of 0.9% Sodium Chloride which functions as a coolant, since drill bits tend to heat up at high RPMs. Speed and torque were seperately tunable on the main module of the device, which the surgeons really liked because exact quality of the bone could not be determined until tactile assessment.
Environment
The operating room for this procedure was unique in the way that communication between sterile and non-sterile fields was critical. The prosthodontist would often consult with implant representatives about proper drill bit sizing and parts to be placed. The representative would take great care in opening the sterile parts (placed to the side of the OR) without compromising the field.
Interactions
This procedure used many forms of computer-aided navigation and measurement approximation. My team and I were able to help out by taping CT images overlayed with the titanium scaffolding and rods on a glass plane, where surgeons could refer back during the surgery. Additionally, a VR representative was present to toggle measurements on and off for specific parts of the procedure. During this part, I noticed that there was particular confusion over what diameter of piece should be used. It was unclear what diameter the surgeons and representatives were talking about since the diameter to drill and the diameter of the implant would be quite different. In fact, one hole was drilled too large, possibly due to this incongruence. Overall, the plastic surgeons, residents and representatives were all impressively educated on each part of the procedure and the mechanics behind the pieces being used, not just the prosthodontist who was placing them.
Objects
As mentioned in interactions, each team member brought something to the table for this operation. Drills, implants, CT scans and typical surgical tool set were all present. There was also a 3D-printed model of the mandible and maxilla CT in the sterile field that the surgeons were able to check the sizing of the implants on and get a better idea of the plane they were working in.
Users
The main user of the drill was the prosthodontist on the case but assistance was needed outside of the sterile field to switch the torque and RPM settings of the tool. I think that, although a small issue, this could could be fixed through installing buttons to toggle the mode on the drill itself or on a separate bluetooth remote that could be kept in the sterile field.
Bad Design – OR Step Stools
Activity
Surgeons come in all shapes and sizes and height is a variable which affects ergonomics and therefore surgeon MSK health. When a team of height-variable surgeons is working together, taller surgeons shouldn’t have to crane their necks in uncomfortable positions to operate but it is equally important that shorter surgeons are at a comfortable level with the OR bed (which does not go down as low as it probably should). To mitigate this discrepancy, bright orange step stools are often used for shorter surgeons (pictured in the middle of the main image).
Environment
I only observed these stools being used by surgeons in the sterile field. I actually also had to stand on top of one once to take a picture of an excised skin flap. The experience was unremarkable but I definitely felt secure standing on top of one.
Interactions
Each time I see these step stools brought into the OR, somebody trips or gets caught on them. It is unclear why this occurs so often, but it’s probably because of their bulky shape and realtive stability (which is necessary for the intended use, but causes problems for surgeons not operating on the stools).
Objects
The orange step stools with a semi-motivational quote about safety.
Users
Shorter surgeons are the primary stakeholders for these step stools. However, they affect the whole team. OR nurses are also responsible for setting them up most of the time so it’s important that their storage is convenient. The small cart they are placed on makes transport easier but I believe these stools should be stackable (people stack and stand on them precariously anyway). I have also seen one surgeon in the past organize the stools all around the OR table. Perhaps if the stool was longer (more like a stage), taller surgeons could put their feet underneath it and minimize trips.
Week 2: Secondary research
QOTW: “In order to make an omelette, we gotta break some eggs.” – [OR 9]
Primary Data Focus
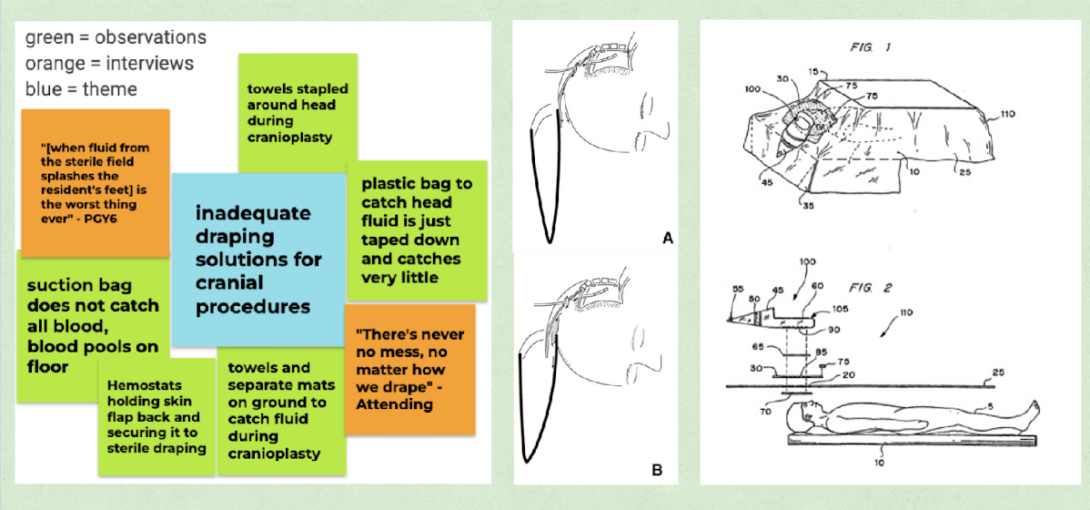
The past two weeks of observation have allowed me to witness several plastic surgery and neurosurgery collaborations where the skull was being manipulated. An example of such a surgery is a craniotomy. This procedure requires a collection pouch that is placed as part of the draping, underneath the patient’s head, and functions to catch fluid from the sterile field and prevent it from pooling on the ground. Primary data listed in the left-most image of the figure encapsulates the potential inadequacies our team observed in this draping.
Literature Review
There was not much literature to be found comparing the collection efficacies of various craniotomy draping designs which was surprising considering the many methods gleaned through conversations with attendings and residents. One article (a “technical note”) explains the importance of fluid catching during craniotomies (generally, patient sterility and surgeon comfort) as well as offers a draping technique that has the bag attached directly to the head of the patient.1 Article figures (in the center of this blog post’s figure) illustrate both the draping method and fluid movement, showing how subtle reinforcements with tape can redirect flow and minimize mess. This is very distinct from draping methods I observed, highlighting the many techniques to draping, which seem to be a personal preference.
Another article from 2018 explored sterility of disposable vs non-disposable surgical drapes. Results showed no significant differences in infection rates between the two types of drapes when investigating their use in orthopedic and spinal surgeries.2 When redesigning surgical drapes, it would be beneficial to consider the eventual translation into a sustainable, reusable drape. Currently, craniotomy fluid collection pouches are often staples for reinforcement, potentially affecting their re-use. An optimal drape would not require these work-arounds.
Patent Review
The image on the right-most side of the blog figure is from a craniotomy drape patent filed by Kimberly Clark Worldwide Inc.3 While this is not the exact model used in my observations, I believe it is actually a slightly better design than what was available in our ORs because of the larger surface area underneath the head. The design’s actual intention seems to be to allow for anesthesiologists to better view the patient’s face. The patent details use of fenestrations, adhesive surfaces and describes material quality (ex: absorbant, ductile) of the draping set and fluid collection pouch combination but does not actually provide information on efficacy or limits of use.
In conclusion, craniotomy drapes optimized for fluid collection could be a worthwhile addition to a surgeon’s armamentarium, but this potential remains understudied and solutions instead rely on provider creativity and perseverance despite discomfort.
Works Cited:
1. Shimizu S, Mochizuki T, Nakayama K, Fujii K. Modified draping to avoid fluid leakage in cranial surgery–technical note. Neurol Med Chir (Tokyo). 2001;41(9):466-468. doi:10.2176/nmc.41.466
2. Kieser DC, Wyatt MC, Beswick A, Kunutsor S, Hooper GJ. Does the type of surgical drape (disposable versus non-disposable) affect the risk of subsequent surgical site infection?. J Orthop. 2018;15(2):566-570. Published 2018 May 7. doi:10.1016/j.jor.2018.05.015
3. https://patents.google.com/patent/US6269815
Week 3: What a need needs
QOTW: “Economy of motion is important… but what eats time is the struggle.” – OR 4
NS 1 Iteration #1 (Tissue de-epithelialization optimization)
A way to address the tissue de-epithelialization process for plastic surgeons that reduces operative time but maintains efficacy.
- Primary Observation: The tissue de-epithelialization process during a pressure ulcer advancement flap was time-consuming without any clear methodology in place apart from using typical surgical instruments (forceps, scissors, scalpel).
- Technical Secondary Research: De-epithelialization has been a critical part of breast reduction surgeries with many solutions studied, including use of the dermatome and VersaJet, which is originally intended for wound debridement.2,3 Neither of these solutions provide consistent thickness and the latter is associated with complications of cyst development needing later procedural intervention.
- Competitors/Influencers and TAM: VersaJet Hydrosurgery system and EpiCut are the primary competitors on the market. For TAM (total addressable market), breast reductions seem to be the primary surgery despite our initial observations being on a pressure ulcer case. 95,881 reductions (for males and females) were done in 2022, indicating the highest increase in demand for a breast-related plastic surgery since the pandemic.4 However, there are certainly more procedures to be included in this number, although it is currently unclear how often they are incorporated into the workflow of other procedures. More primary and secondary data needs to be obtained to answer these questions and provide a more substantial TAM.
NS 1 Iteration #2
A cost-effective way to improve the tissue de-epithelialization process for plastic surgeons and surgical trainees that reduces operative time and provides high accuracy and precision.
- Population, opportunity, outcome: Broader population identified with more quantifiable outcomes.
- Rationale for changes: More specificity was needed for the population, as not only plastic surgeons may be using it, as in my primary observations. The process was first observed being done by an M4 student on sub-I. Additionally, a team member pointed out that the EpiCut device is not used due to cost-constraints. Surgeon incorporation of the VersaJet also suggests that existing tools would be preferred over prohibitively expensive ones. In terms of outcome considerations, I noticed that the literature focuses on lack of consistency between cut methods and a desire for greater reliability, which can be quantified by accuracy and precision.
Works Cited:
1. Yock PG. Biodesign. Cambridge University Press; 2015.
2. Juma A, Miller JG, Laitung JK. Pedicle deepithelialization in breast reduction and mastopexy using the electric dermatome. Plast Reconstr Surg. 1995;95(1):216-217. doi:10.1097/00006534-199501000-00066
3. Chopra K, Folstein MK, Slezak S, Silverman R, Singh D, Gastman BR. The VersaJet for Breast-Reduction Surgery: Operator Beware. Eplasty. 2012;12:e11.
4. 2022 Plastic Surgery Statistics Report by the American Society of Plastic Surgeons
Week 4: Framing and crafting
QOTW: “Just because something is hard, doesn’t mean you can’t do it” – first year resident
Last week, we were left with the following needs statement pertaining to de-epithelialization:
A cost-effective way to improve the tissue de-epithelialization process for plastic surgeons and surgical trainees that reduces operative time while providing high accuracy and precision.
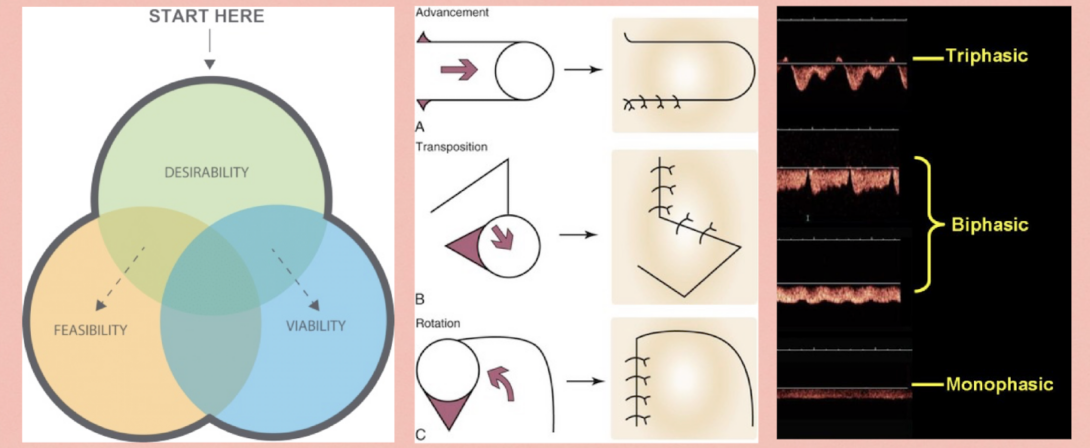
For this week, I’ll be focusing on the IDEO approach to design thinking which emphasizes the need considerations of desirability, feasibility and viability. This is essentially the foundation to human-centered design and is illustrated in the figure above as a Venn diagram; an ideal design would fall in the center, with elements of all three lenses.
NS1 Iteration #3
A cost-effective, practical way to improve the tissue de-epithelialization process for plastic surgeons and surgical trainees that significantly reduces operative time while providing uniform depth thickness and full completion of epidermal excision.
- Desirability: Cost-effectiveness and ease of use are identified as current burdens to either the gold standard (lack of practicality, complex and inconsistent) or competing solutions (expensive). Additionally, even relatively small reductions in operative times, even as little as 9 minutes, could provide substantial savings and freeing of operative times for other procedures.1
- Feasibility: Technical feasibility pertains to the function of the solution we have for our need. While the need statement from last week only used accuracy and precision, more thought was needed to parse out the actual issues in existing solutions. It seems like the primary complication from improper de-epping is inclusion cyst formation which is a result of leftover epidermis. Thus, full excision of the epidermis is required as well as a consistent thickness of the excision. The middle image in the figure provides an example of the types of flaps where this is most needed, as sometimes subcutaneous implantation of advancement flaps is needed for skin preservation.
- Viability: Economic viability may be estimated with the time-saving feature in our anticipated solution. The average OR cost per minute is $30-$38 and the average time for skin de-epithelialization to be completed is estimated to be around 30 minutes for lesser experienced surgeons and about half that for surgeons who routinely perform this process (from primary interviews).2 Even a decrease in procedure length of 5 minutes could mean a savings of $150, although it would subjectively be ideal to cut de-epithelialization length in half for both experienced and novice skill levels.
In order to keep building on all primary data that has been coalesced, I have also came up with one additional need statement for this week.
NS 2 Iteration #1 (Artery-vein differentiation)
Surgeons utilizing acoustic doppler for intraoperative artery vein differentiation need a way to simplify subjective sound output of the device which results in less OR noise and more objective decision-making.
- Primary Observation: Handheld acoustic dopplers are commonly used to identify a vessel as an artery as opposed to a vein. This is based on a whooshing sound heard through the doppler’s speaker which is a transduction of the vasculature’s waveform. Different types of doppler waveforms may be seen in the above figure, where most veins provide a monophasic form and most arteries provide a bi or triphasic form.This method seems unintuitive and just another sound in the OR that the surgeon has to determine what the output means.
- Technical Secondary Research: Many methods exist for this differentiation with the major limitation being cost. CT angiography is the best choice for surgical planning but standby acoustic doppler reasonably corroborates this standard.3
- Competitors/Influencers and TAM: There are 47.8 free flap reconstructions for head and neck per center per year in the US4 and there are approximately 5,828 surgical centers in the US. There is a $28,460 mean cost of these surgeries.5 Thus, the market for this need (just head and neck free flap reconstructions in this example) is 47.8*5,828*$28.460 = $7.928 M This is a gross underestimation since doppler is used for all free flaps and even in transplants surgeries and in the clinic.
flap picture: https://plasticsurgerykey.com/flaps/
IDEO picture: https://medium.com/juanpacheco/ideo-human-centered-design-hcd-design-process-a27eae26e3da
doppler forms picture: https://www.researchgate.net/figure/HV-waveform-patterns-in-patients-with-PHT_fig1_51500527
Works Cited:
1. van den Heuvel J, Does RJ, Bogers AJ, Berg M. Implementing Six Sigma in The Netherlands. Jt Comm J Qual Patient Saf. 2006;32(7):393-399. doi:10.1016/s1553-7250(06)32051-x
2. Childers CP, Maggard-Gibbons M. Understanding Costs of Care in the Operating Room. JAMA Surg. 2018;153(4):e176233. doi:10.1001/jamasurg.2017.6233
3. Hallock G. G. (2011). Acoustic Doppler sonography, color duplex ultrasound, and laser Doppler flowmetry as tools for successful autologous breast reconstruction. Clinics in plastic surgery, 38(2), 203–211. https://doi.org/10.1016/j.cps.2011.03.001
4. Weckx A, Loomans N, Lenssen O. Perforator free flaps in head and neck reconstruction: a single-center low-volume experience. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(4):429-435. doi:10.1016/j.oooo.2016.11.010
5. Kroll SS, Evans GR, Goldberg D, et al. A comparison of resource costs for head and neck reconstruction with free and pectoralis major flaps. Plast Reconstr Surg. 1997;99(5):1282-1286. doi:10.1097/00006534-199704001-00011
Week 5: Stories of decisions and pain
QOTW: “You could do that… or you could change medicine” – OR 5
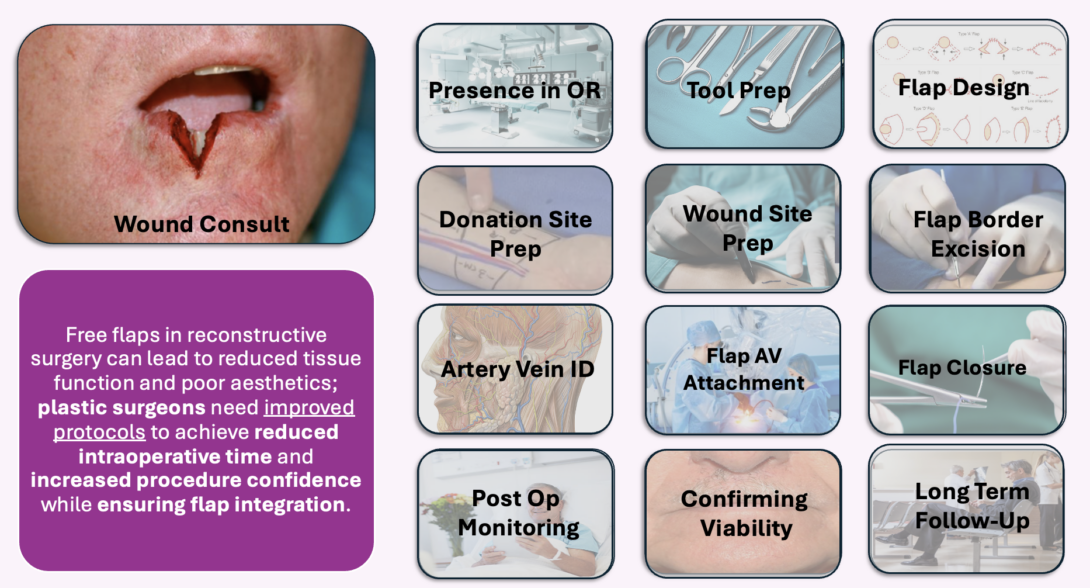
This week, my team and I took a deeper look at the timeline of a free flap procedure. In the OR, I was able to observe (part of a 12 hour surgery) where a full thickness flap from the back was used to reconstruct a face deformity caused by previous excision of a basal cell carcinoma. This procedure also involved harvesting of part of the scapula (incredible!!). Below are some helpful definitions for understanding the intricacies of this storyboard:
- Pain point – a fault in either a tool or method in the story causing frustration, needing workarounds or consuming excess material (time or physical)
- Decision tree – point in the story where the lead stakeholder has multiple avenues to choose from
- Branching point – a potential outcome of a decision tree which takes the story off track and onto a different path
The first decision tree of the story is introduced at the wound consultation. For lip wounds, if the defect is less than 1/3 of the lip width, primary closure via suture is enough. If this is the decision made, the rest of the free flap storyline is rendered obsolete which saves time and money. Thus, primary closure in this context may be seen as a branching point.
Flap design planning is where I identified quite a complex decision tree. The surgeon must consider many factors in flap design including qualities of the wound site (previous of planned irradiation, infection) and donor site (available volume, pliability). Oftentimes, attendings will be discussing two or three ways they are thinking to carry out the flap as the patient is getting prepped.
If a free flap is decided on, the artery vein identification step is a major microsurgical pain point that I also described in last week’s post. Here, the acoustic handheld doppler is a seemingly inadequate solution for providing surgeons with confidence about their flap pedicle flow state. One attending has even said that “the dopplers here are universally terrible.”
During flap closure, we run into a potential branching point also related to last week’s need of de-epithelialization improvement. If too much tissue is taken but could be used to reinforce the flap, de-epping may be done. As we now know, this skill is not done without frustration and has it’s own complications of increased operative time and potential for cyst formation.
Week 6: Final (bittersweet) thoughts
Murphy’s law: anything that can go wrong, will.
For each day I spent observing in the operating room this summer, I lived four lifetimes. The Plastic and Reconstructive Surgery team at UICOM has taught me so much these last six weeks about not only their expansive field but also what it means to be a good teammate, student and collaborator. I feel incredibly grateful for the quality of mentorship in this program and for the hard work of my teammates, especially my IMED colleague Mia! At the end of my undergraduate career, I remember being involved in a research project centered around flap monitoring. At the time, I researched the procedure as best as I could but now know that truly nothing compares to firsthand observation.
CIP allowed me to meet many different stakeholders in the OR — patients, surgeons, residents, nurses and device reps to name a few. However, I feel like most of the needs identified the last few weeks were hardly patient-centered. Of course, they will all eventually benefit the patient by outcome improvement but I can’t help wondering what other avenues in plastic surgery could be explored to create a direct, appreciable impact on the patient: something that would empower them and promote their healing. Many of the final products of the surgeries I observed won’t be seen for months. Or worse, they’ll need to be redone in a few months. The experience of the patient within this timeframe is a pain point I was unable to explore during the program but frequently wonder about. Some of the disfigurements I observed in clinic were devastating to the point of no reasonable intervention being available. Others were hardly noticeable. In the future, I would like to learn more about patient satisfaction with their reconstructive procedures in the long term.
To future CIP participants: introduce yourself to everyone, take good notes and be intentional. A lot of the ideas my team and I came up with were based on hearing the conversations between professionals, not even observing the actual procedure. Even if you don’t feel useful in the room, there’s a lot of information that can be gathered in your role. Additionally, try not to confuse flaps and grafts (a flap has a vascular supply, a graft does not).