Ameera Lodhi
Ameera Lodhi

Basic Information about Student
I am a senior Bioengineering student with a concentration in imaging. I am also on the Pre-Medicine track.
Blog
New criteria
After brainstorming, we made concept cards of our ideas. We had three options utilized in four different devices: collecting tears using capillary action, mechanical suction, or motorized suction. After laying out the benefits of each idea, we presented Dr. Jain with our concepts. He was very eager to see how a motorized option would work since he knew the downside of capillary action and mechanical suction from practice. From that session with Dr. Jain, we came up with some more criteria and, using that, eliminated all but one idea which we are currently trying to refine and prototype.
The new criteria we developed from the meeting with Dr. Jain are:
- Anything that touches the eye should be soft
- Anything collecting tears should be detachable and easily replaceable
- The material touching the collected tears should be non-adherent to proteins and water
Requirements
After more refinement, our final need statement reads:
“To create a more efficient tear collection process, increasing accessibility, while maintaining a safe and comfortable experience for patients.”
This new statement encapsulates the need for a more widespread method of tear collection as well as a more pleasant experience for the patient. With a finalized need statement, we could brainstorm some potential solutions that fall into the criteria we chose. These criteria were selected based on requirements in our need statement that seemed broad, including “efficient,” “accessibility,” “safe,” and “comfortable.”
For efficiency, our requirements were that the tear collection process:
- Should be done within one minute
- Should collect 50 microliters of tear sample from each eye in a healthy patient with no dry eye conditions
For accessibility, we chose specifications which would spread the user beyond specialists and ophthalmologists, including the requirements that:
- Healthcare professionals should be able to do it after one use
- Should be handled by one person without additional materials (such as a slit lamp)
For safety, we specified that:
- The new process should not cause injury to the eye
- Materials that touch the eye should be sanitary
- Materials which come in contact with tears should be disposable
Last, our comfort criteria was that:
- Anything in the eye should remain in the eye for less than 10 seconds at a time
Based on these specifications, we brainstormed potential solutions using the “crazy 8” method. Then, we each chose at least one of those solutions and did some additional research as to how it could work. All of our potential solutions which fit the above criteria were single, handheld devices.
Needs statement
The assignment for this week was to write a needs statement based on problems we saw in our time shadowing Dr. Jain. At the end of this week, after multiple revisions, our final needs statement was:
To create a more user-friendly tear collection process that allows any healthcare professional with minimal training to collect patients’ tears safely
My original needs statement read: “To make the tear collection process more accessible for clinicians and patients with limited resources.”
After consulting as a group, we changed it to: “To modify the tear collection process to make it user friendly for more healthcare workers to use safely on patients.”
This is because, although the population affected would include both the patient benefiting from tear collection and the healthcare worker who would be utilizing the process, we decided to limit it to the professional’s use of the device and the patient’s safety since those are the primary concerns we would have when improving on the process.
However, the statement did not yet encapsulate the ease of use of the process. To illustrate that, we added “minimal training.”
Storyboarding
This week’s observation and interviews provided insight into the tear collection process. Using this information, we developed a storyboard and highlighted potential pain points.
Found Objects
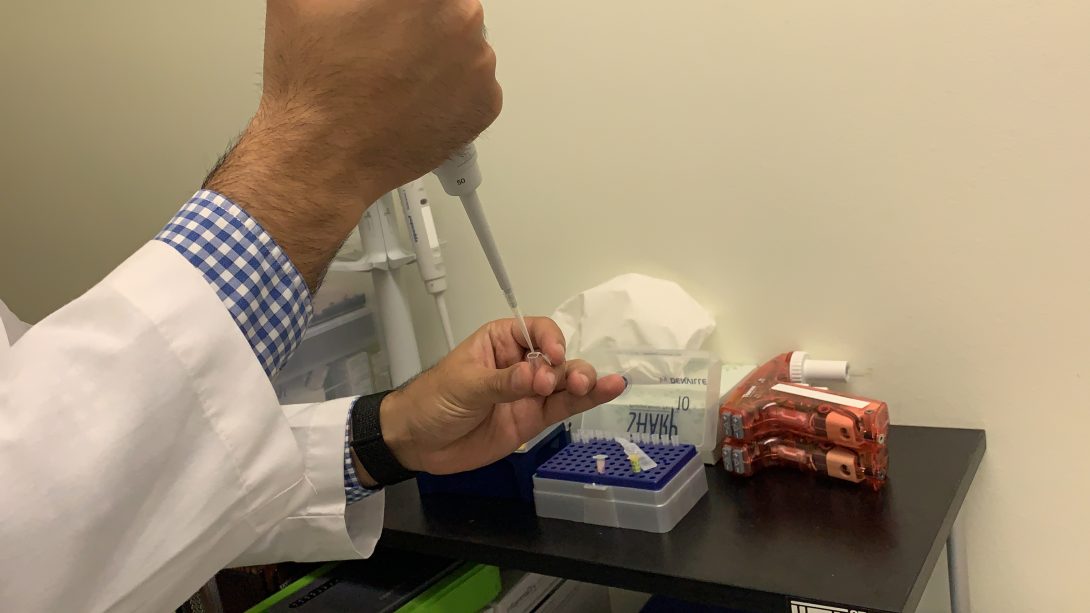
The first step is the preparation, which includes the collection of many “found objects.” For instance, micropipettes are prepared with 5 microliters of artificial tears for each eye. Also, capillary tubes are used to collect the tears from the lower eyelid, and a pipette controller is used to dispense the tears from the capillary tubes into Eppendorf tubes.
User "Torture"
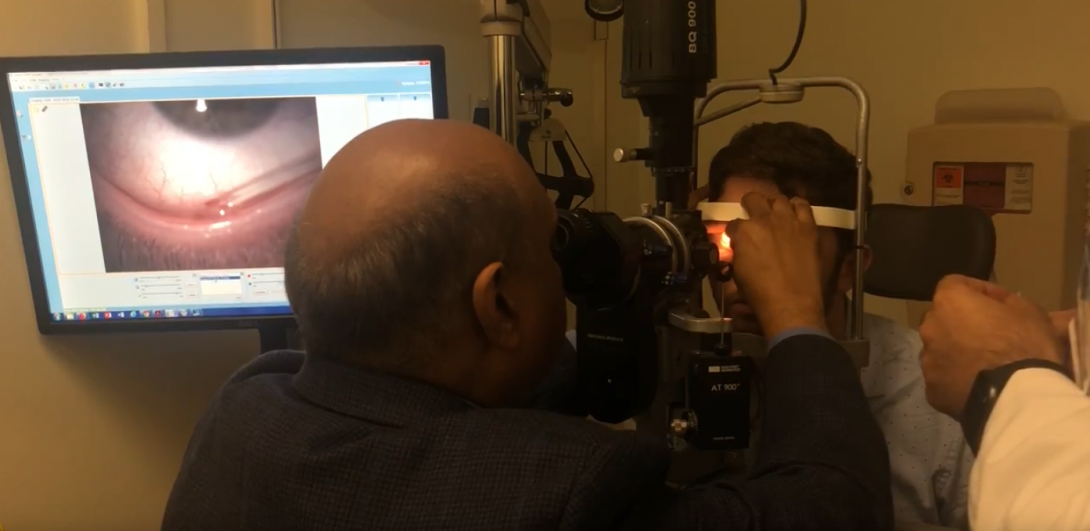
The next steps involve Dr. Jain dispensing the tears in the eye, the research assistant timing the distribution of the tears, Dr. Jain’s collection of the tears, and the research assistant handing the empty capillary tubes to Dr. Jain and retrieving the full ones for testing. There are many pain points in these steps for 3 of the users: the doctor, the research assistant, and the patient.
During all these steps, the doctor is reaching around the slit lamp and holding the eyelid in order to ensure the pipette and capillary tubes are placed in the correct location to safely dispense and collect the tears. This is laborious, as seen from the photo above, but a necessary measure due to the risk associated with placing fragile glass so close to the eye.
It is also necessary for the research assistant to exhibit caution when handling the full capillary tubes. During another interview with an assistant, they mentioned the possibility of breaking a tube and losing an irretrievable sample from patients with tear deficiencies.
Patient interviews were used to illustrate pain points for the patients. While established patients have full confidence in Dr. Jain and don’t think twice about tear collection, new patients mention the difficulty of seeing glass approach their eye and wanting to blink during collection. Additionally, during an interview with a research assistant, we were informed that use of lab equipment, specifically micropipettes, has scared some patients.
last steps

The last steps of the process solely involve the research assistants. They dispense the tears from the capillary tubes to Eppendorf tubes using a pipette controller. These are taken to the lab for further testing. The tears from one of the capillary tubes is dispensed into an MMP-9 test, which then indicates if the patient is positive or negative for inflammation.
First Day on the Job
During our first week in the clinic we shadowed Dr. Jain while he saw his dry eye patients in the Eye and Ear Infirmary.
Good Design
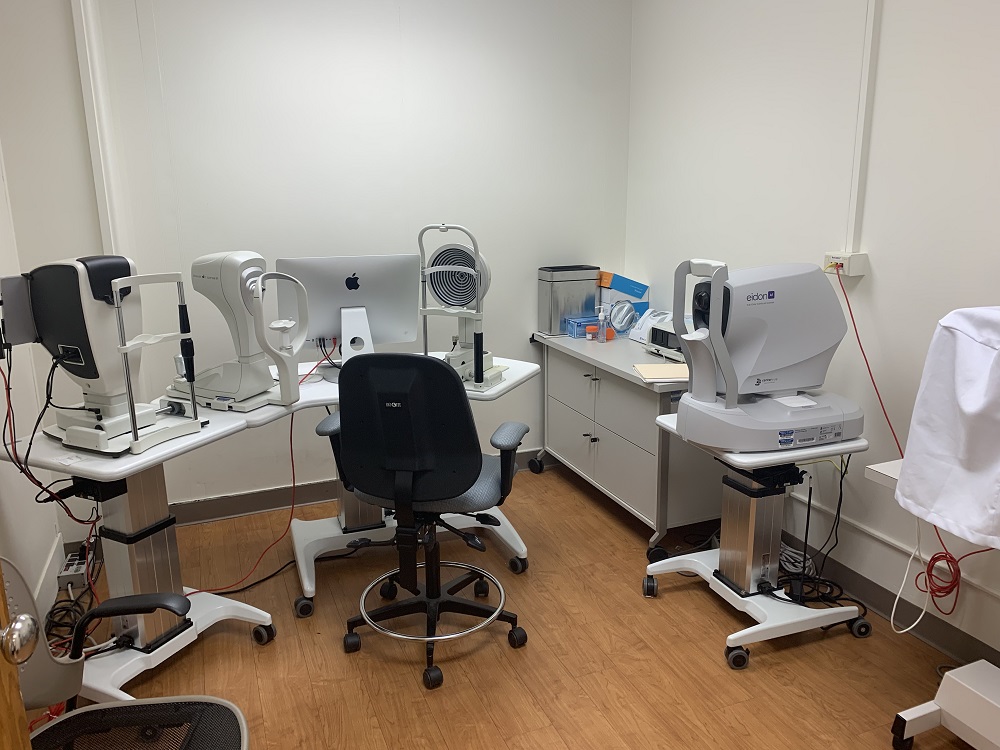
The best design was seen in the rooms with the imaging equipment. Many of these machines are quite user-friendly. In regards to the patient, support is provided so they can remain still while being photographed. The imaging machines also provide simple instructions and feedback when the images can’t be analyzed and another picture needs to be taken, so the physician or lab assistant can use it with ease as well.
One machine in particular which shows good design is the LipiView® II Ocular Surface Interferometer with Dynamic Meibomian Imaging™ (DMI). For this, the users did not need to worry about alignment or blinking because it captures 20 seconds of video where the patient blinks normally. The video is then used to measure lipid layer thickness. It also provides information on partial blinks. Last, the research assistant would flip the eyelid to see if there’s any damage to the meibomian glands. This makes the LipiView the easiest and most useful machine, and, thus, the best-designed.
Bad Design
Due to the availability of the room shown above and the time it takes to run these tests, not all patients were seen here, and rarely by Dr. Jain. One test he conducted with every patient was vital staining. This is where Dr. Jain places dyes in the patient’s eye and qualitatively grades their irritation based on where and how much the dye collects. This is done to track the patient’s progress during treatment, but Dr. Jain mentioned that it gives very little information on the patient’s condition, providing no insight into the pathophysiology of their dry eye.
The process for grading the eye is long as well. Using a micropipette, Dr. Jain places 5 µL of 2% Fluorescein in the patient’s eyes, then waits 2 minutes. Using a slit-lamp, he examines corneal staining. After the 2 minutes, he adds another 5 µL of 1% lissamine green to each eye and grades the staining again. If time permits and the photography slit-lamp is available, he also takes photographs of the staining patterns. After this, one of Dr. Jain’s lab assistants removes the dye using saline drops and a napkin. As the drops are placed in the eye, the lab assistant places the napkin at the bottom of the patient’s face and continuously dabs. This takes another 2 minutes, making the process a total of 5-7 minutes.
Vital staining would be considered bad design because it is qualitative and grading can be inconsistent, especially between physicians. It would be more reliable if pictures can be quickly taken for each visit, but that is not possible due to time constraints and a lack of space and resources. Therefore, this test provides little information while taking most of the patient’s 15-minute appointment time and 2 people (Dr. Jain and a lab assistant) to complete.